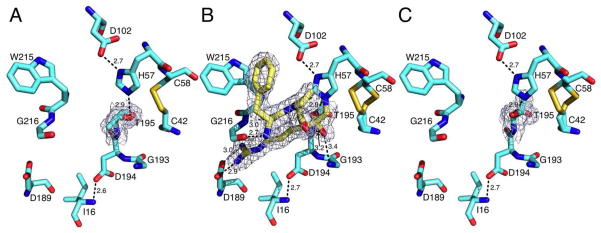
Figure 1.
Active site conformations of the thrombin mutant S195T free (A) or bound to PPACK (B,C) as determined by x-ray crystallography (Table 1). The side chain of T195 positions the methyl group toward an incoming substrate and the Oγ atom in the opposite direction but 3.1 Å away from the C42-C58 disulfide bond. This defective conformation of the nucleophile is corrected upon binding of PPACK (B, yellow sticks) that makes all the expected contacts with residues within the active site. The PPACK-bound conformation (C, PPACK removed for clarity) has an orientation of the Oγ atom of T195 compatible with substrate acylation. Shown are relevant H-bonding interactions. PPACK makes the expected contacts with the N atoms of T195 and G193 in the oxyanion hole, the N and O atoms of G216 and the carboxylate of D189, as well as a strong edge-to-face interaction with W215 in the aryl binding site. The presence of T195 does not alter the critical H-bonding interactions between D102 and H57, or the ion-pair between D194 and the N-terminus I16 that stabilizes the active site and oxyanion hole. There is no steric clash in the two orientations of T195 with residues within the active site. The distance of the methyl group from H57 is 4.1 Å in the free form (A) and 3.2 Å in the PPACK-bound form (C). Electron density (green mesh) is a 2F0-Fc map contoured at 1.0 σ.