Table 1. Optimization of the Arylalkynyl Sulfone Synthesis.
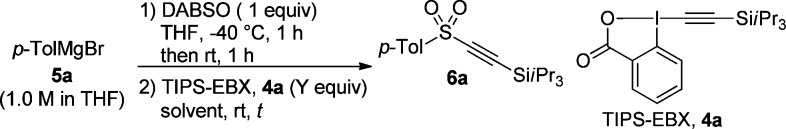
| entrya | equiv of 4a | solvent | t | yield (%)d |
|---|---|---|---|---|
| 1 | 1.2 | DMFb | 2 h | 16 |
| 2 | 1.2 | DMFb | 14 h | 0 |
| 3 | 1.2 | DMF/H2Ob,c | 2 h | 15 |
| 4 | 1.2 | DMF/H2Ob,c | 14 h | 0 |
| 5 | 1.2 | DMF | 2 h | 65 |
| 6 | 1.2 | DMSO | 2 h | 0 |
| 7 | 1.5 | DMF | 2 h | 50 |
| 8 | 1.5 | DMF | 1 h | 50 |
| 9 | 1.5 | DMF | 30 min | 75 |
| 10 | 1.5 | DMF | 5 min | 78 |
| 11 | 1.2 | DMF | 5 min | 80 |
| 12 | 1.1 | DMF | 5 min | 75 |
0.06 mmol p-tolylmagnesium bromide (5a) was used in 0.2 mL of THF. 0.2 mL of solvent was added for the second step (final concentration: 0.13 M).
THF was removed before adding 0.2 mL of solvent (final concentration: 0.3 M).
Ratio (v/v) = 5/1.
The yield was obtained based on 1H NMR analysis using 1,3,5-trimethoxybenzene as internal reference.
