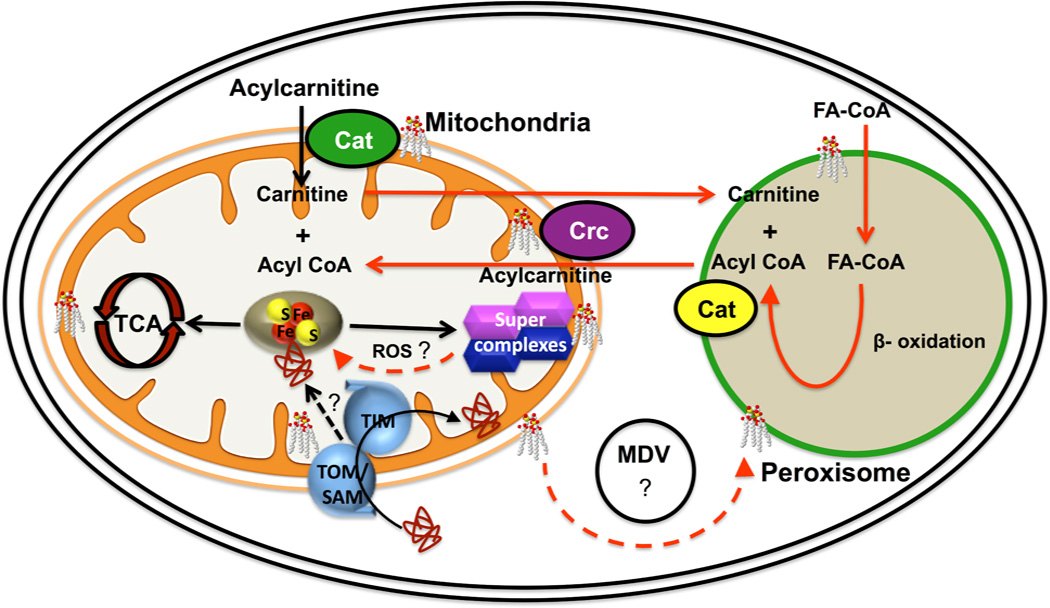
Figure 2. Functions of cardiolipin (CL) in metabolic pathways.
CL is most abundant in the inner membrane and is also present in the outer membrane of mitochondria. It is required for activities of transporters and electron transport chain enzymes and for stabilization of electron transport supercomplexes. Loss of CL leads to perturbation of Fe-S biogenesis, resulting in decreased activity of Fe-S enzymes in the TCA cycle, electron transport, and other pathways. The mechanism linking CL and Fe-S biogenesis is unknown. Because CL is required for the import of proteins through mitochondrial import complexes (TOM, SAM and TIM), it is possible that import of specific proteins required for Fe-S synthesis is defective in CL deficient cells. Alternatively, increased ROS generated by inefficient electron transport in CL deficient cells may damage Fe-S proteins. CL is also present in the membrane of the peroxisome, an organelle that carries out β-oxidation of fatty acids, ether lipid synthesis, and reactions of the glyoxylate cycle. The route whereby CL is transported from mitochondria to peroxisomes is unclear, but may involve mitochondria derived vesicles (MDVs). Acyl CoA produced by β-oxidation of long chain fatty acids in peroxisomes is transported to the mitochondria via the carnitine shuttle. The acyl CoA is transferred to carnitine in the peroxisome by carnitine acyltransferase (Cat). Acylcarnitine from the peroxisome crosses the mitochondrial membrane, facilitated by the carnitine/acylcarnitine translocase (Crc). CL is required for efficient activity of both mitochondrial carnitine enzymes in mammalian cells.