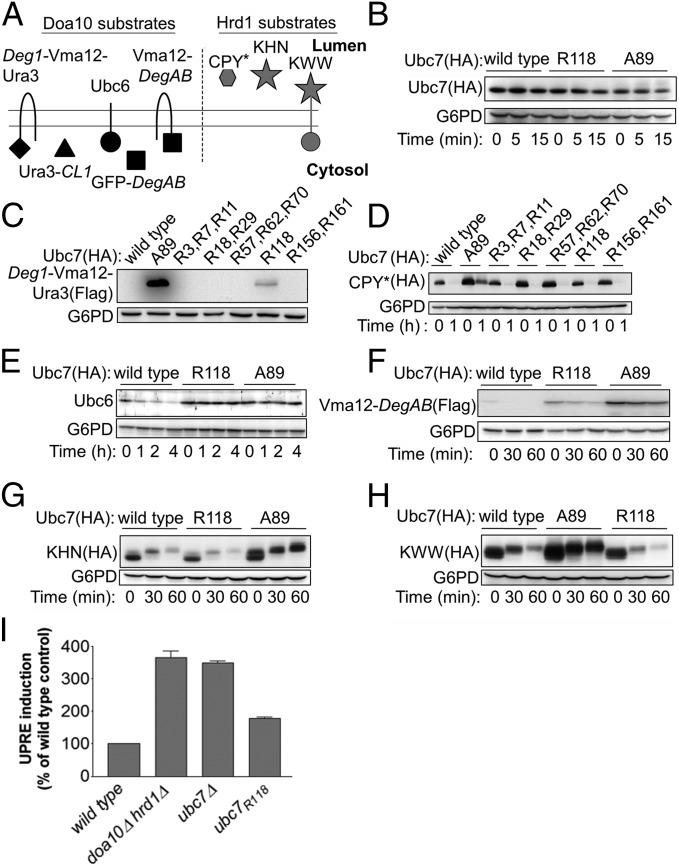
Fig. 3.
Ubc7R118 differentially influences the in vivo degradation of Doa10 and Hrd1 substrates. (A) Schematic presentation of the different ERAD substrates used in this study. (B) Determination of Ubc7 stability in cells expressing wild-type Ubc7, Ubc7A89, or Ubc7R118. Cells at logarithmic phase were collected at time 0 or after incubation with CHX for the indicated time periods and were lysed by incubation with 0.1 N NaOH for 5 min, followed by boiling in sample buffer containing 50 mg/mL DTT. Ubc7 was visualized by immunoblot using anti-HA Abs. (C–H) Determination of the stability of the indicated Doa10 and Hrd1 substrates (shown in A) in cells expressing wild-type Ubc7, Ubc7A89, or Ubc7R118. Cells were treated and as described in B. Proteins were visualized by immunoblotting with anti-Ubc6 Abs, anti-FLAG Abs (for visualizing Flag-tagged Deg1-Vma12-Ura3 and Vma12-DegAB), or anti-HA Abs (for visualizing CPY*, KHN, or KWW). In all experiments G6PD staining was used for loading control. (I) Induction of UPR in Ubc7 mutant cells. The indicated cells, expressing a LacZ reporter under the control of the UPR-activated Kar2 promoter (UPRE-LacZ), were lysed, and β-galactosidase steady-state levels were determined by an activity assay using ortho-Nitrophenyl-β-galactosidase as a substrate. Data shown are the mean ± SE of three or more independent experiments.