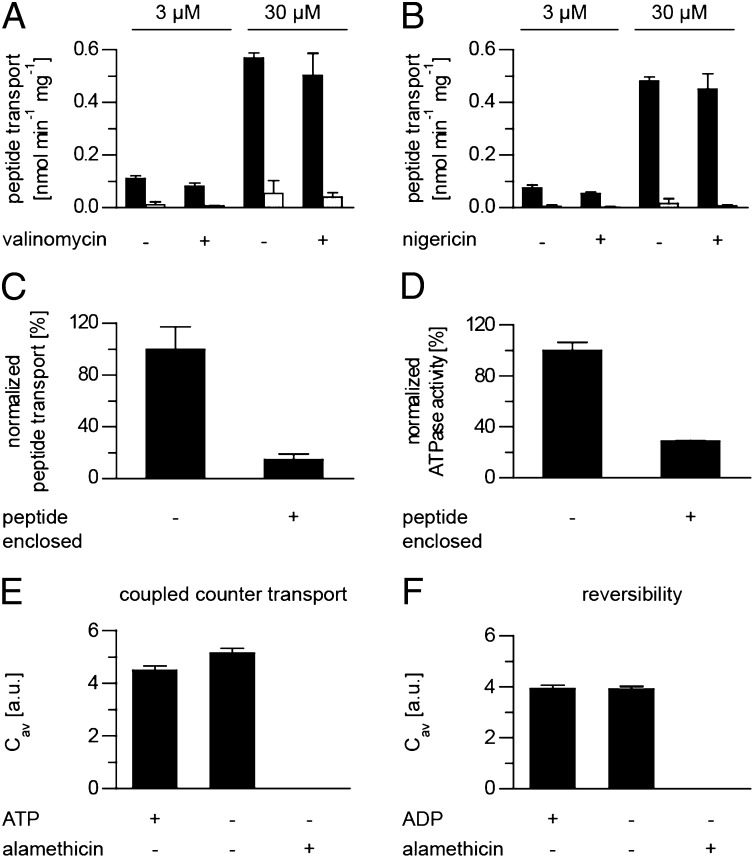
Fig. 5.
Peptide accumulation by TAPL is limited by trans-inhibition and is unidirectional. (A and B) Proteoliposomes (50 µg lipid, 2.5 µg coreTAPL) were incubated in 140 mM KPi, pH 7.5, containing peptide RRYCATTO488KSTEL (3 or 30 µM) with (black) or without (white) Mg-ATP (3 mM) at 37 °C for 60 min in the presence or absence of either 1 µM valinomycin (A) or nigericin (B). (C) Proteoliposomes (2.5 µg coreTAPL, 50 µg lipid), empty or prefilled with 3 mM unlabeled peptide (RRYQKSTEL), were incubated with 3 mM Mg-ATP and 3 µM RRYCATTO488KSTEL at 37 °C for 15 min. Background fluorescence measured in the absence of Mg-ATP was subtracted. The transport assay was performed in triplicate, with error bars indicating SD. (D) ATPase activity of proteoliposomes (2 µg coreTAPL, 40 µg lipid), empty or prefilled with 3 mM unlabeled peptide (RRYQKSTEL), was measured for 15 min at 37 °C in the presence of 3 mM Mg−ATP. The ATPase assay was performed in triplicate, with error bars indicating SD. (E and F) TAPL acts as strictly unidirectional ABC exporter. Proteoliposomes prefilled for 20 min at 37 °C in the presence of 3 µM RRYCATTO488KSTEL were incubated with either 60 µM unlabeled peptide RRYQKSTEL in the presence or absence of Mg-ATP (E) or were incubated in phosphate buffer (20 mM) containing 3 mM Mg−ADP (F), and peptide efflux was analyzed by DCFBA over 10 min at 37 °C. As control, addition of alamethicin (9.8 µM) induced instantaneous peptide efflux. Laser powers were kept constant: 488, 3 µW; 633, 1 µW.