Abstract
Predators can impact their prey via consumptive effects that occur through direct killing, and via non-consumptive effects that arise when the behaviour and phenotypes of prey shift in response to the risk of predation. Although predators' consumptive effects can have cascading population-level effects on species at lower trophic levels there is less evidence that predators' non-consumptive effects propagate through ecosystems. Here we provide evidence that suppression of abundance and activity of a mesopredator (the feral cat) by an apex predator (the dingo) has positive effects on both abundance and foraging efficiency of a desert rodent. Then by manipulating predators' access to food patches we further the idea that apex predators provide small prey with refuge from predation by showing that rodents increased their habitat breadth and use of ‘risky′ food patches where an apex predator was common but mesopredators rare. Our study suggests that apex predators' suppressive effects on mesopredators extend to alleviate both mesopredators' consumptive and non-consumptive effects on prey.
Keywords: non-lethal effect, indirect effect, Notomys fuscus, landscape of fear, giving up density, trophic cascade
1. Introduction
Predators can impact their prey and smaller predators (mesopredators) via two mechanisms: consumptive (i.e. lethal) effects that occur through direct killing and non-consumptive (i.e. non-lethal) effects that become manifest as prey and competitors shift their phenotypes and habitat use in response to risks associated with predation [1,2]. The consumptive effects of predators, by moderating the consumptive effects that herbivores and smaller predators have on their prey, can induce trophic cascades whereby predators' effects propagate through ecosystems and have alternating positive and negative population-level effects on species at lower trophic levels [3]. For example, predation of herbivores by apex predators can suppress their populations, reducing grazing pressure, and thus result in increased biomass of the preferred forage species of herbivores [4,5].
Most studies investigating the non-consumptive impacts of predators have investigated their effects on the behavioural, physiological, morphological and life-history traits of their prey and competitors [6–9]. Such studies typically show that prey and competitors at risk of being killed by a predator shift their behaviour to reduce predation risk and undergo associated shifts in metabolism and the quality and quantity of food ingested [6]. These non-lethal effects of predators can be translated to the demography of herbivores and mesopredators if they impair the reproduction and longevity of individuals [10].
If predators' consumptive effects can propagate cascades of population-level effects and induce non-lethal effects in their prey, it follows then that they should also be capable of propagating cascades of non-consumptive effects [11]. Such cascades of non-consumptive effects may arise if apex predators moderate the risk that smaller predators pose to their prey, and thus may be expected to alternate with trophic level in a manner analogous to the consumptive effects of predators.
The mesopredator release hypothesis (MRH) proposes a trophic pathway through which apex predator removal can dramatically alter community structure [12,13]. This hypothesis posits that the absence of apex predators ‘releases' smaller mesopredators from predation and/or competition constraints placed on them by apex predators, and in doing so facilitates increased mesopredator abundance [14]. Hyper-abundant mesopredators may then prey heavily on, and suppress the abundances of, animal species that fall below the weight range normally preyed on by apex predators [13,15,16].
Mesopredator release pathways have classically been described using lethal effects models, whereby the frequency of fatal encounters between mesopredators and prey, and hence population-level impacts of mesopredators, are reduced in the presence of an apex predator. However, it is conceivable also that lower encounter rates between mesopredators and prey in the presence of an apex predator should reduce the risk of predation perceived by small prey species [17]. Such a refuge effect might be expected to become manifest as prey species reducing their vigilance and allocating more time to foraging in environments where apex predators are common and mesopredators rare [18].
Giving up density (GUD) trials use enriched food patches to titrate the relative influence that food and ‘safety′ have in determining foraging animals' allocation of time, taking the amount of food left uneaten in foraged food patches as a proxy for ‘fear′ [19,20]. Foraging theory predicts that a foraging animal will cease to forage in a food patch when the perceived benefit of continuing to exploit the patch is outweighed by the perceived risk [19]. Thus, low GUD values (a low density of food remaining in patches) are expected in low-risk areas where animals forage more efficiently by foraging food patches more thoroughly [20]. Conversely, high GUD values are expected in high-risk areas where diminishing rates of return become outweighed by the risks associated with continuing to forage. When replicated spatially, GUDs can be used to map ‘landscapes of fear′, which seek to explain how animals exploit food and habitat resources across heterogeneous foraging environments [21,22].
In this paper, we investigate how foraging behaviour (food patch using; GUD) and habitat exploitation in a desert rodent, Notomys fuscus, are influenced by the activity levels of an apex predator, the dingo (Canis dingo), two mesopredators, the red fox (Vulpes vulpes) and the feral cat (Felis catus), moon phase and conspecific abundance. We conducted our study in the Strzelecki Desert, Australia. In this region, dingoes, although a predator of small mammals, provide a net benefit for N. fuscus populations by suppressing the abundance of red foxes [15,18]. These benefits accrue because red foxes are more likely than dingoes to predate upon N. fuscus [18]. There is also evidence that direct killing by dingoes can suppress the abundance of the other introduced mesopredator in the region, the feral cat (F. catus) [23]. Like foxes, feral cats prey more heavily on small mammals than dingoes do [5].
Applying the mesopredator release hypothesis and our a priori knowledge of interactions thought to occur between dingoes, mesopredators and N. fuscus, we tested two hypotheses concerning how dingoes may influence food patch and habitat use of N. fuscus. First, because predation risk is expected to reduce the amount of time individuals allocate to foraging, we predicted that the GUD of N. fuscus will be lower in areas where dingoes are common because the risk of predation is lower owing to dingoes' suppressive effects on mesopredator populations and activity. Second, because risk of predation can reduce foraging animals' use of ‘risky’ habitats [24], we predicted that the breadth of habitat used by N. fuscus should be greater in areas where dingoes are common because the risk of predation is lower [25,26]. In addition to the above hypotheses, because conspecific density dependence can potentially increase animals' allocation of time to foraging and increase the range of habitats exploited owing to intra-specific competition and/or ‘safety in numbers' effects [27,28], we also predicted that the GUD of N. fuscus should be lower and the breadth of habitat use greater in areas with higher N. fuscus population densities.
We conducted two experiments to test our predictions. We conducted a landscape-scale GUD trial and used structural equation modelling (SEM), in conjunction with abundance and activity estimates for predators and N. fuscus, to explore if variation in the activity levels of an apex predator influenced the GUD of N. fuscus. To further parse out the effects that predators and conspecifics had on the foraging efficiency of N. fuscus, we then conducted a manipulative cover experiment to compare how N. fuscus exploited ‘risky′ and ‘safe′ food patches in response to variation in the activity levels of an apex predator, mesopredators and conspecifics.
2. Material and methods
(a). Study site
The study was conducted on rangeland properties in the Australian states of South Australia and Queensland that are used for grazing cattle at low densities (less than 0.1 to 2.85 cattle km−2; figure 1). The predominant landforms in the Strzelecki Desert are longitudinal sand dunes. Mean annual rainfall in the study area ranges from 188 to 227 mm [29]. Vegetation on sand dunes is dominated by an understorey of ephemeral grasses, forbs and herbs (less than 40 cm) and a sparse overstorey of perennial shrubs. The study was conducted following a prolonged period of high rainfall associated with La Niña phase of the El Niño Southern Oscillation.
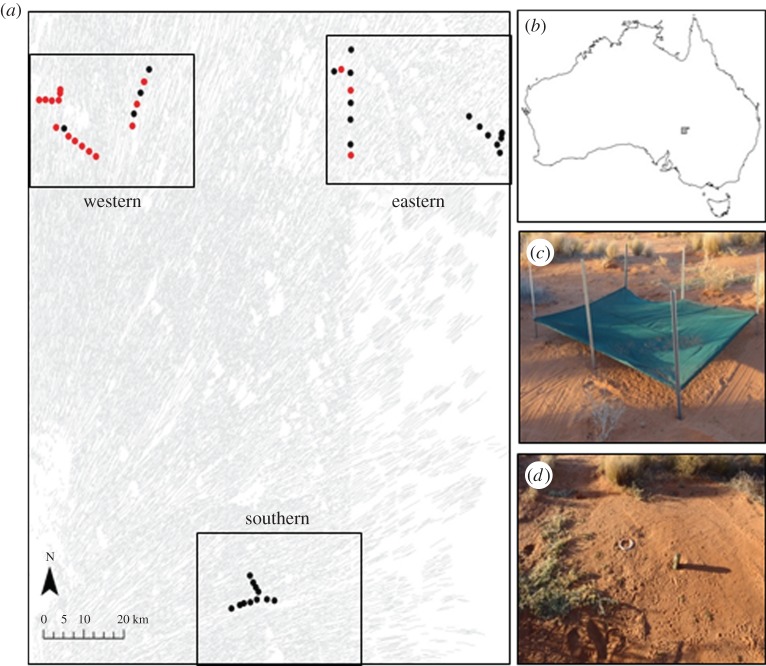
Figure 1.
(a) Map showing the study area in the Strzelecki Desert of central Australia. Black circles represent sites used in experiment 1; red circles represent sites used in both experiments 1 and 2. Rectangular polygons represent the eastern, western and southern regions of the study area. Underlying grey lines represent longitudinal sand dunes. (b) Location of the study area (grey polygon) within Australia. (c) A photograph of the covered, ‘safe′ treatment areas used in experiment 2. (d) A photograph of the open, ‘risky′ treatment areas used in experiment 2.
Dingoes are relatively common in the study region [15,18,30]. Because dingoes may kill calves, some landholders in the eastern region of the study area (figure 1) control dingo populations using meat baits impregnated with the poison 1080 (sodium monofluoroacetate) and shooting. However, in the western and southern regions of the study area (figure 1) no dingo control is undertaken other than opportunistic shooting. Previous studies show that fox and cat abundance and activity typically increases in areas where dingo populations are suppressed because they are released from direct killing and interspecific competition by dingoes [31]. The variation in dingo abundance resulting from the different levels of dingo control used in our study area provided a natural experiment to evaluate the effects that dingoes, and in turn mesopredators, had on the foraging behaviour of N. fuscus.
(b). Dingo, fox, cat and Notomys fuscus abundance
We conducted nocturnal spotlight surveys to determine if dingo, fox, cat and N. fuscus abundance indices varied between the western (120 km), eastern (136 km) and southern (241 km) regions of the study area (figure 1). Spotlight surveys are suitable for indexing the abundances of predators and N. fuscus abundance in the study area because the sparse vegetation allows for long lines of sight [18]. Spotlight surveys were conducted along single-lane dirt roads during 2007 (east and south regions only), 2012, 2013 and 2014. All surveys commenced at dusk. During spotlight surveys, dingoes, foxes, cats and N. fuscus were counted by an observer using a 50 W spotlight while sitting on the roof (approx. 2.3 m above ground level) of a four-wheel-drive vehicle moving at 15 km h−1. Indices of dingo, fox, cat and N. fuscus abundance were calculated as the number of individuals observed during a spotlight transect divided by the length of each transect.
Generalized linear mixed-effects models (Poisson-link function) were used to compare the indexed abundance of dingoes, foxes, cats and N. fuscus between east, west and south regions of the study area (figure 1), where sample period was treated as a random factor. An offset was added to all models to account for differences in sampling effort between study regions.
(c). Experiment 1: the effects of predators and conspecific abundance on the GUD of Notomys fuscus
We undertook a landscape-scale study to determine how the GUD of N. fuscus varied in response to variation in dingo, fox and cat activity, and the abundance of N. fuscus. We did this by conducting GUD trials using enriched patches of narrow-leaved hopbush (Dodonaea viscosa angustissima; henceforth ‘hopbush′) seed at 47 sites subject to differing levels of predator control between May and October 2012 (figure 1).
Before conducting our experiment, we constructed an a priori SEM derived from interaction pathways theorized to occur between dingoes, foxes, cats, N. fuscus, moon phase and vegetation cover (figure 2). Dingo activity was predicted to negatively affect both fox and cat activity through direct killing and interference competition [31]. In turn, dingo activity was predicted to be correlated negatively with the GUD of N. fuscus by decreasing the risk of N. fuscus individuals being killed by a fox or cat [18]. Fox and cat activity was predicted to negatively affect N. fuscus abundance through predation [15]. In turn, fox and cat activity were predicted to have a positive correlation with the GUD of N. fuscus because the risk of N. fuscus individuals being killed by a predator should increase with increasing mesopredator activity [18]. Notomys fuscus abundance was expected to negatively affect N. fuscus GUD because conspecific density dependence can potentially increase animals' allocation of time to foraging and increase the range of habitats exploited owing to intra-specific competition and/or ‘safety in numbers' effects [27,28]. Moon phase at the time of the study was expected to positively affect the GUD of N. fuscus because previous studies show that rodents perceive a greater risk of predation with increasing moonlight [32]. Vegetation cover was used as a proxy measure for food availability (N. fuscus consumes the seeds, stems and leaves of grasses, herbs and forbs [33]) and was predicted to positively affect the GUD of N. fuscus because food availability is known to be a primary factor influencing the ‘missed opportunity cost′ of foraging [20].
Figure 2.
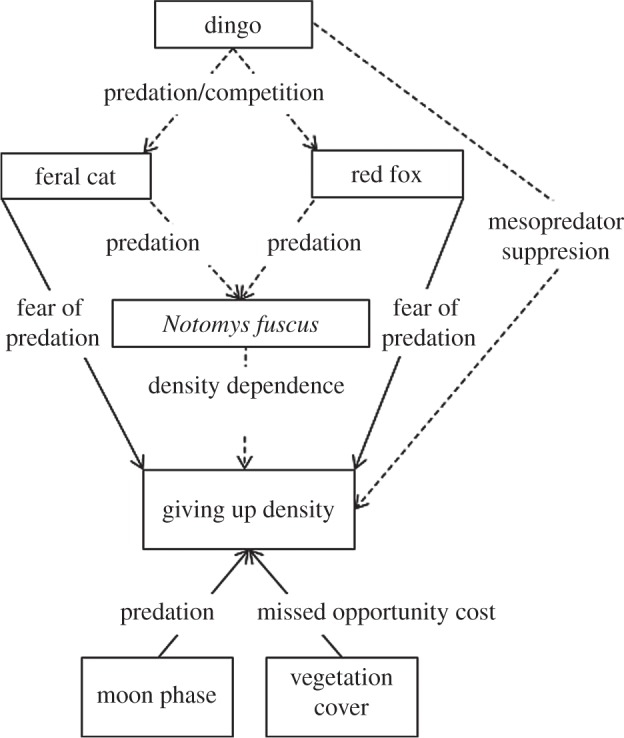
The a priori SEM showing the hypothesized effects that dingo, fox and cat activity, N. fuscus abundance, moon phase and vegetation cover were expected to have on the giving up density of N. fuscus in experiment 1. Solid lines represent positive pathways and dashed lines represent negative pathways. Text dissecting arrows show predicted responses of one variable on another.
Hopbush is the dominant shrub within the study area and N. fuscus is known to consume hopbush seed. At each site, five plastic bowls (15 cm diameter, 5 cm depth) were filled with a matrix of sand and placed at 20 m intervals along a transect extending from dune bottom to dune top areas. Forty hopbush seeds (approx. 2 mm diameter) were added to each seed tray and mixed through the sand before dusk. The following morning, the seeds were recounted and replenished. This procedure was conducted for two to three consecutive days. Sampling over three nights was not always possible owing to logistical constraints imposed by climatic conditions and mechanical breakdowns. To confirm that N. fuscus was consuming seeds from the foraging trays, the sand in a 1 m radius surrounding trays was swept daily. Foraging by N. fuscus was determined by the presence of their distinctive tracks. Only seed trays foraged by N. fuscus were included in analysis. The tracks of sympatric rodent species (Rattus villosissimus, Pseudomys hermannsburgensis, Pseudomys desertor and Mus musculus) were seldom observed in areas surrounding foraging trays (less than 10% of foraging trays). GUD values were calculated for each site as the mean of values recorded across all five trays on the last two sampling nights. The first night of sampling was used to habituate mice to experimental seed trays and was excluded from analysis.
Notomys fuscus abundance at each GUD site was indexed in the 3 days immediately prior to GUD trials by live trapping of mice using metal box traps (H. B. Sherman Traps, Tallahassee, FL, USA) baited with a mixture of peanut butter, oats and golden syrup. Traps were placed at 20 m intervals within a 4 × 5 grid. Trapping was conducted for two to three consecutive nights. Captured animals were given a unique mark with a marker pen. Recaptured individuals were excluded from analyses. Bait was replenished daily. An index of N. fuscus abundance was calculated at each site as the total number of individuals captured per trapping night.
Predator activity was indexed at each site using 40 m track plots located on single-lane dirt roads approximately 2 m wide at the bases of sand dunes. Track plots were swept daily, and the occurrence of dingo, fox and cat tracks was monitored for two to three consecutive days. An index of predator activity was then calculated as the proportion of nights that tracks of each predator were observed at each site.
Moon phase data were obtained from the Australian Bureau of Meteorology and expressed as a percentage (0 indicated a new moon and 100 indicated a full moon).
Ground cover vegetation was measured at each site using a point-step method [34]. At each site, an observer noted the presence or absence of live grasses, herbs and forbs (less than 40 cm height) at 1 m point intervals along three 100 m survey transects. The percentage of ground cover was then calculated for each site.
We used SEM to evaluate support for hypothesized direct and indirect factors influencing the GUD of N. fuscus (figure 2) [35,36]. SEMs use path diagrams and correlative data to infer causal relationships between test variables based on knowledge of biologically relevant interaction between species. Because SEMs calculate a covariance matrix between test variables, indirect path coefficients can be estimated and model reduction techniques can be used for model simplification.
We constructed an a priori SEM (see above section for model justification) and used an accelerated bootstrap method to test our predictions (figure 2) [35]. Accelerated bootstrap methods are appropriate for SEMs with relatively low sample sizes or with non-normal data [35]. The Bollen–Stine (BS) test statistic was used to assess how well the final SEM fit the bootstrapped covariance matrix (i.e. how well the model reproduces the data) [35]. Non-significant BS p-values (α = 0.05) indicated consistency between the replicated bootstrapped distribution and the data, and are required for the interpolation of causal pathways within SEMs [35].
We used backward step-wise model reduction to simplify our model [37]. Non-significant pathways were sequentially deleted from the a priori model until all coefficient estimates were significant. The significance of direct path coefficients within this ‘most parsimonious' SEM was then assessed by comparing estimated values against the bootstrapped distributions, and indirect path coefficients were calculated by multiplying all direct path coefficients along indirect pathways [35]. Only path coefficients with significant (p < 0.05) correlations are presented in the results and figures. Analyses were conducted in the computer program IBM SPSS AMOS (Armonk, NY, USA).
Because spatial dependence is a problem within many landscape-scale studies [38], we tested for spatial autocorrelation of our dependent variable (the GUD of N. fuscus) using Moran's I-test on the residuals of a generalized linear model (Poisson-link function) which contained all variables used in our full SEM model. Data are not spatially autocorrelated if the Moran I-statistic tends towards 0 and the associated p-value is non-significant (α = 0.05)
(d). Experiment 2: the effects of predator activity and conspecific abundance on habitat use by foraging Notomys fuscus
Because the results of a pilot study and preliminary findings from experiment 1 suggested that both predator activity and conspecific abundance influenced the GUD of N. fuscus (see Results), we conducted a manipulative experiment to parse out their effects by comparing the exploitation of food patches by N. fuscus in adjacent ‘risky′ and ‘safe′ habitats. The rationale for this experiment was that where N. fuscus perceived greater risk of predation, they should forage more from ‘safe′ sheltered habitats than ‘risky′ open habitats [19,24]. To conduct our experiment, we established 18 pairs of ‘risky′ and ‘safe′ food patches across a gradient of predator activity (figures 1 and 3) and measured the difference in the GUD between ‘risky′ and ‘safe′ pairs, as well as the activity of predators and abundance of N. fuscus. We used the difference in the GUD between ‘risky′ and ‘safe′ sites within each pair, measured as the log response ratio (LnRR) [39], as our response variable. By using the LnRR, a standardized metric of effect size, we were able to measure the relative allocation of foraging effort by N. fuscus in ‘safe′ versus ‘risky′ food patches while controlling for the effects that conspecific abundance and predator activity had on the GUDs that were evident in the results of experiment 1. If dingoes provided N. fuscus with refuge from predation by mesopredators, we predicted that the difference in the GUD between ‘risky′ and ‘safe′ patches should be similar in areas of relatively low predation risk, where dingoes are common and mesopredators rare. Conversely, we expected that N. fuscus should forage more from ‘safe′ patches, and thus the difference in the GUD of ‘risky′ and ‘safe’ patches should be greater in areas of high predation risk, where dingoes are rare and mesopredators common. If conspecific abundance was an important determinant of the GUD of N. fuscus, we predicted the difference in the GUD between ‘risky′ and ‘safe′ patches should decrease with increasing population density.
Figure 3.
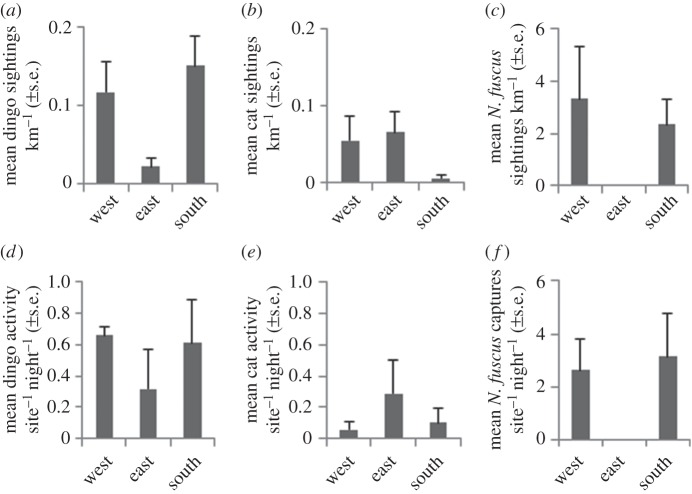
Column graphs comparing mean (±s.e.) (a) dingo sightings km−1, (b) cat sightings km−1, (c) N. fuscus sightings km−1, (d) dingo activity site−1 night−1, (e) cat activity site−1 night−1 and (f) N. fuscus captures site−1 night−1 between east, west and south regions of the study area.
Paired ‘safe′ and ‘risky′ food patches were established in open areas on dune tops at 18 of the 47 replicate sites (six sites sampled during July 2012, 12 sites sampled during August 2012) used in experiment 1 described above. ‘Safe′ patches were constructed by suspending a 2 × 2 m shade cloth (Coolaroo shade cloth, www.coolaroousa.com) 20 cm above the ground using wire and metal fence posts (figure 1). The shade cloth was suspended low to the ground so that it would impede the access that predators, but not N. fuscus, had to the foraging trays. Hence, the safe patches were designed to provide foraging N. fuscus with a refuge habitat to which predators had difficulty accessing in comparison with ‘risky′ patches situated in open areas. To confirm that predators were excluded from safe patches, the track plots in a 1 m radius surrounding the foraging trays (see above) were monitored for predator tracks. Dingo, fox and cat tracks were never observed on track plots located under ‘safe′ treatments, confirming that ‘safe′ treatments successfully excluded predators. ‘Risky′ patches were placed in open areas devoid of vegetation spaced 5 m from ‘safe′ patches and consisted of 4 metal fence posts marking a similar 2 × 2 m area (figure 1). Experimental treatment blocks were constructed at least three days before experiments were conducted. One tray containing 50 hopbush seeds was placed at the centre of each ‘safe′ and ‘risky′ patch at each site and sand surrounding seed trays was swept daily. The GUD of N. fuscus was then assessed for three consecutive nights (for methods see §2c).
Dingo, fox and cat activity was assessed using the same 40 m predator tracking plots used in experiment 1. To increase the detection rate of predators, an additional 40 m tracking plot was located on dune top areas surrounding experimental blocks. An index of dingo, fox and cat activity was calculated as the total activity between dune top and bottom areas per sampling night. Live trapping data from experiment 1 were used to estimate N. fuscus abundance at each replicate site. ‘Safe′ and ‘risky’ patches used in experiment 2 were located 50–100 m from live trapping grids to reduce spatial confounding.
The risk perceived by foraging N. fuscus was assessed by calculating the difference in the GUD of paired ‘safe’ and ‘risky′ food patches using the LnRR, with ‘safe′ food patches as the numerator and ‘risky′ patches as the denominator [39]. Positive LnRR values denoted that N. fuscus consumed more seed from ‘risky′ patches than ‘safe’ patches (i.e. GUD higher at ‘safe′ treatments than ‘risky′ treatments). Negative LnRR values denoted that N. fuscus consumed more seed from ‘safe′ patches than ‘risky′ patches (i.e. GUD higher at ‘risky′ patches than ‘safe’ patches).
We used a linear mixed-effects model to assess the relative importance that dingo, fox and cat activity, and N. fuscus abundance (henceforth ‘predictor variables′), had on the LnRR of N. fuscus GUD. Sampling period was treated as a random factor. All predictor variables were standardized to have a mean of 0 and a standard deviation of 1 prior to model fitting. We tested for spatial autocorrelation within our response variable using a Moran's I-test on the residuals of the linear mixed-effects model. Linear mixed-effects models were conducted in the computer program R [40] using the nlme [41] library.
3. Results
Dingoes were detected most frequently in the western and southern study regions during spotlight (figure 3; Wald χ2 = 9.035, d.f. = 2, p = 0.011) and track plot surveys (average activity per region from tracking plot sites used in experiment 1; figure 3a,d). Cats were detected most frequently in the eastern and western study regions during spotlight surveys (Wald χ2 = 7.582, d.f. = 2, p = 0.023) and in the eastern study region during track plot surveys (figure 3b,e). Foxes were never detected on spotlight surveys and rarely detected on track plot surveys (average = 0.037 ± 0.027 s.e. plots disturbed night−1).
Notomys fuscus was commonly detected to the west and south of the study region, and never detected to the east of the study area on both spotlight (χ2 = 7.834, d.f. = 2, p = 0.020) and formal track plot surveys (average activity per region from tracking plot sites used in experiment 1; figure 3c,f). Although N. fuscus was never detected to the east of the study region, their presence was confirmed by the presence of tracks on sand dunes and their remains in predator scats.
(a). Experiment 1: the effects of predators and conspecific abundance on the GUD of Notomys fuscus
The most parsimonious SEM explaining the GUD of N. fuscus contained all variables except moon phase and vegetation cover (figure 4). Dingo activity was correlated negatively with cat activity (−0.50), and fox (−0.18) and cat (−0.39) activity were correlated negatively with N. fuscus abundance (figure 4). Because dingoes had a negative effect on cat activity and cat activity had a negative effect on N. fuscus abundance, dingo activity had an indirect positive effect on N. fuscus abundance (−0.50 × −0.39 = 0.20; figure 4).
Figure 4.
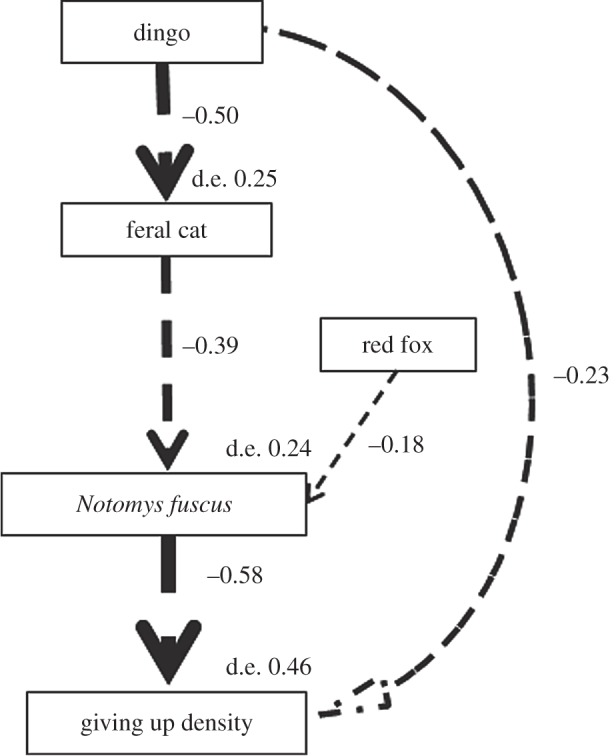
The most parsimonious SEM explaining the giving up density of N. fuscus in experiment 1. Dashed arrows represent negative pathways and are weighted by standardized path coefficient estimates. The SEM suggests that cat activity was higher where dingo activity was low; that N. fuscus abundance was higher where cat and fox activity were low and dingo activity was high; and that the giving up density of N. fuscus increased with increasing dingo activity and N. fuscus abundance. d.e., deviance explained by each variable.
Dingo activity (−0.23) and N. fuscus abundance (−0.58) were correlated negatively with the GUD of N. fuscus (figure 4; electronic supplementary material, figure S1). Thus, increases in dingo activity and N. fuscus abundance resulted in decreased ‘fear′ responses of N. fuscus. Fox and cat activity had no direct effect on the GUD of N. fuscus (electronic supplementary material, figure S1). However, cats had an indirect positive effect on the GUD of N. fuscus because cat activity had a negative effect on N. fuscus abundance (−0.39 × −0.58 = 0.23; figure 4). Hence, increases in cat activity and associated decreases in N. fuscus abundance resulted in increased ‘fear′ responses of N. fuscus. Dingoes had an indirect negative effect on the GUD of N. fuscus because dingo activity had a negative effect on cat activity, and cat activity had a negative effect on N. fuscus abundance (−0.50 × −0.39 × −0.58 = −0.11; figure 4). No spatial autocorrelation occurred within the residuals of the response variable used in our SEM (Moran I = 0.006, p = 0.638).
(b). Experiment 2: the effects of predator activity and conspecific abundance on habitat use by foraging Notomys fuscus
The linear mixed-effects model revealed that N. fuscus consumed more seed from ‘safe′ patches than ‘risky′ patches where cat activity was high, but took similar amounts of seed from ‘safe′ and ‘risky′ patches where dingo activity was high (table 1). This was because dingoes had a strong positive effect (0.646) on the LnRR of N. fuscus GUDs, and cats had a strong negative effect (−0.391) on the LnRR of N. fuscus GUDs (table 1). These results suggest that N. fuscus individuals altered their behaviour to minimize encounters with cats more than with dingoes. Notomys fuscus abundance and fox activity were not present within the most parsimonious linear mixed-effects model (table 1), and thus had a negligible influence on patch use by N. fuscus. No spatial autocorrelation occurred within the residuals of the response variable in the linear mixed-effects model (Moran I = 0.019, p = 0.399). Collinearity was low between all predictor variables (electronic supplementary material, table S1).
Table 1.
Results of a linear mixed-effects model comparing the effects that the activity of dingoes, foxes and cats, and Notomys fuscus abundance had on the log response ratio (LnRR) of giving up densities of N. fuscus in adjacent ‘safe′ and ‘risky′ foraging patches measured in experiment 2. Negative LnRR values indicate a preference for sheltered habitats and positive LnRR values indicate a preference for open habitats.
| predictor variable | coefficient estimate | F-statistic | p-value |
|---|---|---|---|
| dingo | 0.646 ± 0.113 | 32.56 | <0.001 |
| red fox | 0.232 ± 0.132 | <0.001 | 0.992 |
| feral cat | −0.391 ± 0.109 | 7.572 | 0.020 |
| Notomys fuscus | 0.170 ± 0.149 | 2.967 | 0.135 |
4. Discussion
Our study provides evidence that mesopredator suppression by an apex predator can alleviate the risk of predation perceived by a small prey species. This refuge effect was evidenced by results showing that (i) where dingoes were common, cats were relatively rare; (ii) N. fuscus were more abundant where dingoes were common and cats were rare; (iii) N. fuscus foraged less apprehensively where dingoes were common and cats were rare; and (iv) N. fuscus foraged more apprehensively in open versus sheltered habitats where dingoes were rare and cats common, but showed similar levels of apprehension between sheltered and open habitats where dingoes were common and cats were rare. Viewed collectively our results provide evidence that apex predators' suppressive effects on mesopredators propagate to both population-level and behavioural effects on the prey of mesopredators.
Contrary to our first prediction and a wealth of literature suggesting that dingoes have strong suppressive effects on fox activity throughout much of arid and semi-arid Australia [31], dingoes appeared to have a negligible effect on fox activity in our study. The absence of a correlation between dingo and fox activity in this study may have been owing to the relatively high abundances of dingoes, and hence top-down pressure on fox populations, throughout the study area. This interpretation is supported by previous studies and our spotlight survey data showing consistently high dingo activity and consistently low fox activity within the immediate study area between 2007 and 2014 [18].
Consistent with the mesopredator release hypothesis, dingo activity was correlated negatively with cat activity. Previous studies have suggested that dingoes, even though they kill and sometimes eat cats [23,42], do not always have a strong negative correlation with cat abundance and activity [31]. In northern regions of the continent, where foxes are absent, dingoes appear to suppress the abundance and activity of cats [43,44]. However, indices of dingo and cat abundance show negative, neutral and even positive associations in arid and temperate areas where foxes are common [5,31,45]. One explanation put forward to explain the variability in the numerical relationships between dingo and cat abundance indices is that both dingoes and foxes have suppressive effects on cat populations [31]. This is supported by studies in North America that have shown complex inter-predator relationships between wolves (Canis lupus), coyotes (Canis latrans) and red foxes [46]. If this was the case, the rarity of foxes within our study area may have released cats from suppression by foxes, allowing cat activity to increase to a level where dingoes' suppressive effects on them could be detected.
In accord with the mesopredator release hypothesis, our results revealed a positive correlation between dingo activity and N. fuscus abundance, and negative correlations between N. fuscus abundance and the activity of cats and foxes. Previous studies suggest that this refuge effect exists because the per capita rate of predation on N. fuscus is reduced in the presence of dingoes [18]. Such an effect may arise because where dingoes are not controlled they tend to occur at lower population densities than foxes and cats do in areas where dingoes have been removed, and because dingoes are less likely to prey upon small mammals than foxes or cats [5,31].
The results of our GUD experiments are consistent with the idea that an apex predator can shape the ‘landscape of fear′ for small prey by reducing the risk of predation. In our landscape-scale GUD trial (experiment 1), the GUD of N. fuscus was correlated positively with cat activity but correlated negatively with dingo activity. This finding suggests that N. fuscus individuals dedicated more time to anti-predator behaviour than feeding behaviour where cat activity was high and dingo activity was low. This presumably occurred because dingoes suppressed cat activity, and in doing so reduced the predatory risk that cats posed to N. fuscus. However, conspecific abundance was also correlated negatively with the GUDs, suggesting that density-dependent factors also influenced the foraging behaviour of N. fuscus (see below). Our manipulative cover experiment, which compared rodents' allocation of foraging effort to adjacent ‘risky′ and ‘safe′ food patches, furthers the idea that dingoes provide N. fuscus with refuge from predation by showing that N. fuscus increased their habitat breadth and made relatively more use of ‘risky′ food patches in areas where dingoes were common but cats rare. Taken together, these findings provide evidence that apex predators' refuge effects can increase the effort that small prey species allocate to foraging, and also increase the breadth of habitats in which prey species choose to forage. Such alleviation of mesopredators' non-consumptive effects could potentially extend to the demography of N. fuscus and contribute to increased abundances, if greater foraging efficiency in the presence of dingoes translates to increased survival and reproductive success.
GUD experiments typically focus on predation risk as being the primary factor influencing foraging behaviour. However, a suite of other factors may also place constraints on foraging, such as resource variability, the energy state of foragers and intra-specific competition [27,28,47,48]. We predicted that, in addition to predation risk, conspecific abundance would influence food patch and habitat use of N. fuscus because individuals should allocate more effort to foraging as population density increases owing to intra-specific competition and/or ‘safety in numbers' effects [27,28]. In support of this prediction, our landscape-scale GUD trials suggested that N. fuscus feed less apprehensively when occurring at high population densities. However, our manipulative cover experiment showed that N. fuscus abundance did not influence food patch exploitation between ‘safe′ and ‘risky′ habitats when N. fuscus density was controlled for in our analyses. Collectively, these results suggest that although N. fuscus abundance was correlated positively with GUDs, food patch use by N. fuscus was primarily a response to the risk of cat predation perceived by foraging individuals.
Supplementary Material
Acknowledgements
Brenton von Takach Dukai provided field assistance.
Ethics statement
Ethical approval was provided by the South Australian Department of Environment and Natural Resources (26/2011) and University of Western Sydney (A8904).
Data accessibility
Data available from Dryad: http://dx.doi.org/doi:10.5061/dryad.hf7gg.
Funding statement
Funding was provided by the Australian Research Council and Margaret Middleton Fund of the Australian Academy of Science.
References
- 1.Lima S. 1998. Nonlethal effects in the ecology of predator-prey interactions. BioScience 48, 25–34. ( 10.2307/1313225) [DOI] [Google Scholar]
- 2.Schmitz OJ. 2008. Effects of predator hunting mode on grassland ecosystem function. Science 319, 952–954. ( 10.1126/science.1152355) [DOI] [PubMed] [Google Scholar]
- 3.Estes JA, et al. 2011. Trophic downgrading of planet earth. Science 333, 301–306. ( 10.1126/science.1205106) [DOI] [PubMed] [Google Scholar]
- 4.Terborgh J, et al. 2001. Ecological meltdown in predator-free forest fragments. Science 294, 1923–1926. ( 10.1126/science.1064397) [DOI] [PubMed] [Google Scholar]
- 5.Letnic M, Koch F, Gordon C, Crowther MS, Dickman CR. 2009. Keystone effects of an alien top-predator stem extinctions of native mammals. Proc. R. Soc. B 276, 3249–3256. ( 10.1098/rspb.2009.0574) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Creel S, Christianson D. 2008. Relationships between direct predation and risk effects. Trends Ecol. Evol. 23, 194–201. ( 10.1016/j.tree.2007.12.004) [DOI] [PubMed] [Google Scholar]
- 7.Schmitz OJ, Krivan V, Ovadia O. 2004. Trophic cascades: the primacy of trait-mediated indirect interactions. Ecol. Lett. 7, 153–163. ( 10.1111/j.1461-0248.2003.00560.x) [DOI] [Google Scholar]
- 8.Werner EE, Peacor SD. 2003. A review of trait-mediated indirect interactions in ecological communities. Ecology 84, 1083–1100. ( 10.1890/0012-9658(2003)084[1083:AROTII]2.0.CO;2) [DOI] [Google Scholar]
- 9.Spencer EE, Crowther MS, Dickman CR. 2014. Risky business: do native rodents use habitat and odor cues to manage predation risk in Australian deserts? PLoS ONE 9, e90566 ( 10.1371/journal.pone.0090566) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Creel S, Christianson D, Liley S, Winnie JA. 2007. Predation risk affects reproductive physiology and demography of elk. Science 315, 960 ( 10.1126/science.1135918) [DOI] [PubMed] [Google Scholar]
- 11.Ripple WJ, Beschta RL. 2004. Wolves and the ecology of fear: can predation risk structure ecosystems? BioScience 54, 755–766. ( 10.1641/0006-3568(2004)054[0755:wateof]2.0.co;2) [DOI] [Google Scholar]
- 12.Ritchie EG, Johnson CN. 2009. Predator interactions, mesopredator release and biodiversity conservation. Ecol. Lett. 12, 982–998. ( 10.1111/j.1461-0248.2009.01347.x) [DOI] [PubMed] [Google Scholar]
- 13.Crooks KR, Soule ME. 1999. Mesopredator release and avifaunal extinctions in a fragmented system. Nature 400, 563–566. ( 10.1038/23028) [DOI] [Google Scholar]
- 14.Prugh LR, Stoner CJ, Epps CW, Bean WT, Ripple WJ, Laliberte AS, Brashares JS. 2009. The rise of the mesopredator. BioScience 59, 779–791. ( 10.1525/bio.2009.59.9.9) [DOI] [Google Scholar]
- 15.Letnic M, Crowther MS, Koch F. 2009. Does a top-predator provide an endangered rodent with refuge from an invasive mesopredator? Anim. Conserv. 12, 302–3012. ( 10.1111/j.1469-1795.2009.00250.x) [DOI] [Google Scholar]
- 16.Johnson CN, Isaac JL, Fisher DO. 2007. Rarity of a top predator triggers continent-wide collapse of mammal prey: dingoes and marsupials in Australia. Proc. R. Soc. B 274, 341–346. ( 10.1098/rspb.2006.3711) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frid A, Baker GG, Dill LM. 2008. Do shark declines create fear-released systems? Oikos 117, 191–201. ( 10.1111/j.2007.0030-1299.16134.x) [DOI] [Google Scholar]
- 18.Letnic M, Dworjanyn SA. 2011. Does a top predator reduce the predatory impact of an invasive mesopredator on an endangered rodent? Ecography 34, 827–835. ( 10.1111/j.1600-0587.2010.06516.x) [DOI] [Google Scholar]
- 19.Brown JS, Kotler BP. 2004. Hazardous duty pay and the foraging cost of predation. Ecol. Lett. 7, 999–1014. ( 10.1111/j.1461-0248.2004.00661.x) [DOI] [Google Scholar]
- 20.Brown J. 1988. Patch use as an indicator of habitat preference, predation risk, and competition. Behav. Ecol. Sociobiol. 22, 37–47. ( 10.1007/bf00395696) [DOI] [Google Scholar]
- 21.Laundré JW, Hernández L, Altendorf KB. 2001. Wolves, elk, and bison: reestablishing the ‘landscape of fear’ in Yellowstone National Park, U.S.A. Can. J. Zool. 79, 1401 ( 10.1139/z01-094) [DOI] [Google Scholar]
- 22.Laundré JW, Hernández L, Ripple WJ. 2010. The landscape of fear: ecological implications of being afraid. Open Ecol. J. 3, 1–7. ( 10.2174/1874213001003030001) [DOI] [Google Scholar]
- 23.Moseby KE, Neilly H, Read JL, Crisp HA. 2012. Interactions between a top order predator and exotic mesopredators in the Australian rangelands. Int. J. Ecol. 2012, 250352 ( 10.1155/2012/250352) [DOI] [Google Scholar]
- 24.Orrock JL, Fletcher RJ. 2014. An island-wide predator manipulation reveals immediate and long-lasting matching of risk by prey. Proc. R. Soc. B 281, 20140391 ( 10.1098/rspb.2014.0391) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hernández L, Laundré JW. 2005. Foraging in the ‘landscape of fear’ and its implications for habitat use and diet quality of elk Cervus elaphus and bison Bison bison. Wildl. Biol. 11, 215–220. ( 10.2981/0909-6396(2005)11[215:fitlof]2.0.co;2) [DOI] [Google Scholar]
- 26.Laundré JW, et al. 2014. The landscape of fear: the missing link to understand top-down and bottom-up controls of prey abundance? Ecology 95, 1141–1152. ( 10.1890/13-1083.1) [DOI] [PubMed] [Google Scholar]
- 27.China V, Kotler BP, Shefer N, Brown JS, Abramsky Z. 2008. Density-dependent habitat and patch use in gerbils: consequences of safety in numbers? Israel J Ecol Evol 54, 373–388. ( 10.1560/IJEE.54.3-4.373) [DOI] [Google Scholar]
- 28.Searle KR, Stokes CJ, Gordon IJ. 2008. When foraging and fear meet: using foraging hierarchies to inform assessments of landscapes of fear. Behav. Ecol. 19, 475–482. ( 10.1093/beheco/arn004) [DOI] [Google Scholar]
- 29.Australia Bureau of Meteorology. 2013. Climate data online See http://www.bom.gov.au/climate/data/?ref=ftr (accessed 28 April 2014).
- 30.Newsome AE, Catling PC, Cooke BD, Smyth R. 2001. Two ecological universes separated by the Dingo Barrier Fence in semi-arid Australia: interactions between landscapes, herbivory and carnivory, with and without dingoes. Rangeland J. 23, 71–98. ( 10.1071/RJ01015) [DOI] [Google Scholar]
- 31.Letnic M, Ritchie EG, Dickman CR. 2012. Top predators as biodiversity regulators: the dingo Canis lupus dingo as a case study. Biol. Rev. 87, 390–413. ( 10.1111/j.1469-185X.2011.00203.x) [DOI] [PubMed] [Google Scholar]
- 32.Daly M, Behrends PR, Wilson MI, Jacobs LF. 1992. Behavioural modulation of predation risk: moonlight avoidance and crepuscular compensation in a nocturnal desert rodent, Dipodomys merriami. Anim. Behav. 44, 1–9. ( 10.1016/S0003-3472(05)80748-1) [DOI] [Google Scholar]
- 33.Murray BR, Dickman CR, Watts S, Morton SR. 1999. The dietary ecology of Australian rodents. Wildl. Res. 26, 857–858. ( 10.1071/WR97046_CO) [DOI] [Google Scholar]
- 34.Landsberg J, James CD, Morton SR, Müller WJ, Stol J. 2003. Abundance and composition of plant species along grazing gradients in Australian rangelands. J. Appl. Ecol. 40, 1008–1024. ( 10.1111/j.1365-2664.2003.00862.x) [DOI] [Google Scholar]
- 35.Grace JB. 2006. Structural equation modeling and natural systems. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 36.Bollen KA. 1989. Structural equations with latent variables. New York, NY: John Wiley and Sons. [Google Scholar]
- 37.Elmhagen B, Ludwig G, Rushton SP, Helle P, Lindén H. 2010. Top predators, mesopredators and their prey: interference ecosystems along bioclimatic productivity gradients. J. Anim. Ecol. 79, 785–794. ( 10.1111/j.1365-2656.2010.01678.x) [DOI] [PubMed] [Google Scholar]
- 38.Dormann CF, et al. 2007. Methods to account for spatial autocorrelation in the analysis of species distributional data: a review. Ecography 30, 609–628. ( 10.1111/j.2007.0906-7590.05171.x) [DOI] [Google Scholar]
- 39.Hedges LV, Gurevitch J, Curtis PS. 1999. The meta-analysis of response ratios in experimental ecology. Ecology 80, 1150–1156. ( 10.1890/0012-9658(1999)080[1150:TMAORR]2.0.CO;2) [DOI] [Google Scholar]
- 40.R Development Core Team 2013. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 41.Pinheiro J, Bates D, DebRoy S, Sarkar D, Team RC. 2013. nlme: linear and nonlinear mixed effects models. R package version 31-111. See http://CRAN.R-project/package=nlme.
- 42.Cupples JB, Crowther MS, Story G, Letnic M. 2011. Dietary overlap and prey selectivity among sympatric carnivores: could dingoes suppress foxes through competition for prey? J. Mammal. 92, 590–600. ( 10.1644/10-MAMM-A-164.1) [DOI] [Google Scholar]
- 43.Kennedy M, Phillips BL, Legge S, Murphy SA, Faulkner RA. 2012. Do dingoes suppress the activity of feral cats in northern Australia? Austr. Ecol. 37, 134–139. ( 10.1111/j.1442-9993.2011.02256.x) [DOI] [Google Scholar]
- 44.Brook LA, Johnson CN, Ritchie EG. 2012. Effects of predator control on behaviour of an apex predator and indirect consequences for mesopredator suppression. J. Appl. Ecol. 49, 1278–1286. ( 10.1111/j.1365-2664.2012.02207.x) [DOI] [Google Scholar]
- 45.Colman N, Gordon C, Crowther M, Letnic M. 2014. Lethal control of an apex predator has unintended cascading effects on forest mammal assemblages. Proc. R. Soc. B 281, 20133094 ( 10.1098/rspb.2013.3094) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Levi T, Wilmers CC. 2011. Wolves–coyotes–foxes: a cascade among carnivores. Ecology 93, 921–929. ( 10.1890/11-0165.1) [DOI] [PubMed] [Google Scholar]
- 47.Morris DW. 1997. Optimally foraging deer mice in prairie mosaics: a test of habitat theory and absence of landscape effects. Oikos 80, 31–42. ( 10.2307/3546513) [DOI] [Google Scholar]
- 48.Wasserberg G, Kolter BF, Morris DW, Abramskey Z. 2007. A field test of the centrifugal community organization model using psammophilic gerbils in Israel's southern coastal plain. Evolut. Ecol. Res. 9, 299–311. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available from Dryad: http://dx.doi.org/doi:10.5061/dryad.hf7gg.