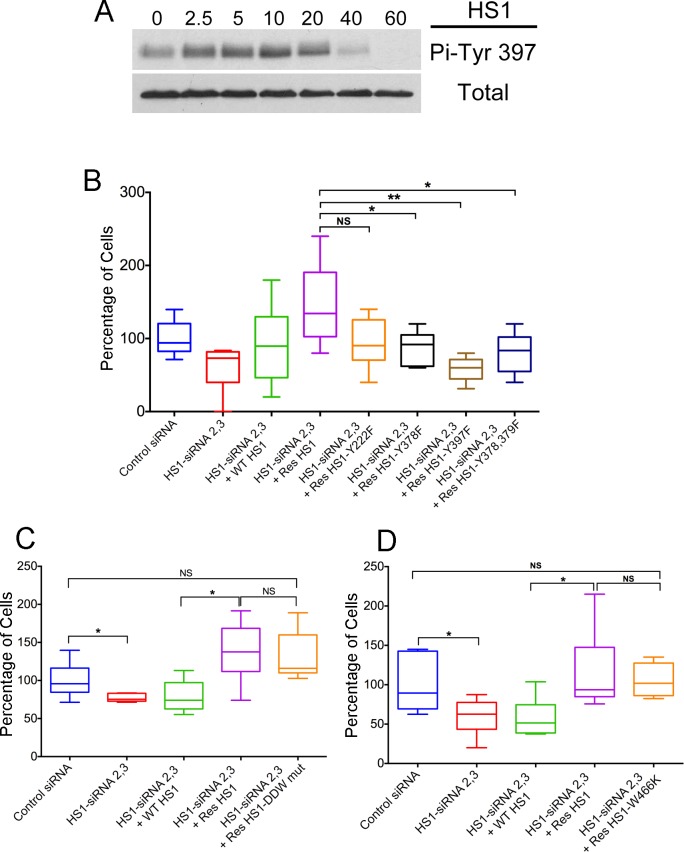
Fig 4. Expression Rescue of HS1 Mutants in HS1-depleted NK Cells.
A) Phosphorylation of HS1 Tyr397 in response to SDF-1α. Immunoblots probed with anti-Phospho-Tyr397 and anti-HS1. NK cells (5 x 106) treated with SDF-1α (30 ng/mL) for the indicated times (min). B—D) Function of HS1 mutants in TEM by transwell assay. Number of cells in the lower chamber, as a percentage of the mean of the control sample value on each day, with box and whisker plots. Boxes: 25th to 75th percentiles; whiskers: minimum and maximum values. B) Mutations of phosphorylated tyrosine residues. Compared to control siRNA (blue), HS1 depletion by siRNA causes decreased TEM (red), and the defect is rescued by expression of wild-type HS1 (green) or siRNA-resistant wild-type HS1 (purple). Expression of siRNA-resistant forms of single-mutant HS1 Y378F (black), single-mutant HS1 Y397F (brown) or double-mutant HS1 Y378F Y397F (dark blue) does not rescue the defect, comparing their values to the value for siRNA-resistant wild-type (purple). Expression of siRNA-resistant HS1 Y222F (orange) rescues with a value that is slightly less, but not statistically significant, from that of siRNA-resistant wild type. Asterisks indicate *P>0.05, **P>0.005 (unpaired Student’s t-test, N = 6–9). C) Mutation of Arp2/3 complex binding site. Expression of siRNA-resistant HS1 with mutation of DDW residues to AAA (orange) rescues the defect, with no difference compared to siRNA-resistant wild-type HS1. N = 6 in each case. D) Mutation of SH3 domain at ligand-binding site. Expression of siRNA-resistant HS1 with the mutation W466K (orange) rescues the defect, with no difference compared to siRNA-resistant wild-type HS1. n = 6 in each case.