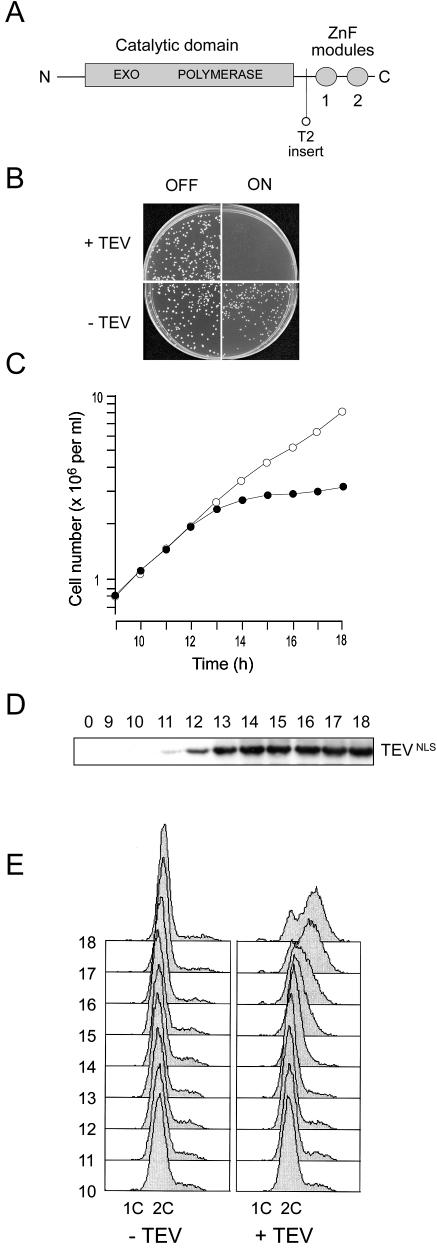
Figure 1.
Removal of the C-terminal zinc finger domain of Sp Pol3 by targeted proteolysis abolishes protein function in vivo. (A) Schematic of the Sp Pol3 protein showing the relative locations of the exonuclease and polymerase motifs in the catalytic domain and the ZnF modules (labelled 1 and 2). Also shown is the location of the TEV site insertion in the Sp Pol3-T2 protein. (B) Induction of TEV in haploid pol3-T2 cells prevents colony formation. Approximately 1000 pol3-T2 cells carrying either pREP3X (indicated as –TEV) or pREP3X-TEVNLS (+TEV) were plated on supplemented EMM plates either with or without thiamine to either repress or depress the nmt1 promoter (labelled OFF or ON). Colony formation was not possible when TEVNLS was induced (top right panel). (C) Induction of TEV in haploid pol3-T2 cells halts cell number increase in liquid culture. TEVNLS expression was induced by transferring pol3-T2 (pREP3X-TEVNLS) cells from thiamine-containing to thiamine-free medium at 32°C at time zero. Cell number increase was monitored from 9 to 18 h using an electronic particle counter (filled circles). As a control, pol3-T2 (pREP3X) cells were analysed in parallel (open circles). (D) Induction of TEVNLS expression. Samples for total protein extraction and immunoblotting were taken from the pol3-T2 (pREP3X-TEVNLS) culture at hourly intervals from 9 to 18 h. TEVNLS induction was apparent from 11 h. (E) Flow cytometric analysis of propidium iodide-stained cells from 10 to 18 h post-induction. Left panel: pol3-T2 (pREP3X) cells. Right panel: pol3-T2 (pREP3X-TEVNLS) cells.