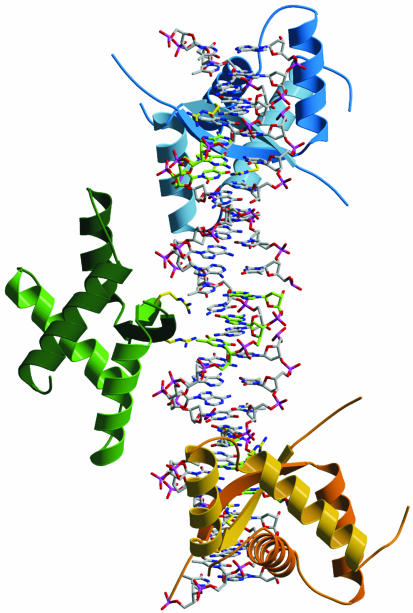
Figure 8.
ω2–DNA complex modeled on the basis of the MetJ–DNA complex. In the latter, two MetJ dimers bind to DNA tandem repeats and the entire lengths of α-helices A of adjacent MetJ are involved in cooperative MetJ–MetJ interactions (32). Ribbon representation of three ω2 proteins [with secondary structure (βαAαB)2] bound to a 25 bp straight B-DNA containing three heptads in direct orientation, —→3 with center-to-center distance 7 bp shown in stick form (5′-A/TATCACA/T-3′, the complementary 3′-AGT-5′ of the central 5′-TCA-3′ were denoted in green). The antiparallel N-terminal β-structure of ω2 binds to the DNA operator half-site and is inserted in the major groove, with R31 and R31′ (their conformation in the complex is not known, indicated by yellow sticks) probably hydrogen bonding to guanine. The βαAαB subunits of each ω2 are colored differently. The α-helices A of adjacent ω2 are so close together that they could interact if the ω2-bound heptads are bent or if ω2 is slightly rotated on the DNA binding site. The ω2 protein bound from seven (Pω) to 10 heptads (PcopS, see Fig. 1) should wrap around the DNA helix.