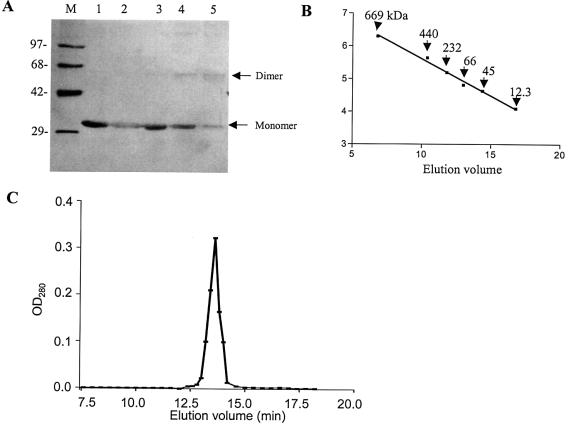
Figure 2.
Subunit structure of KpnI REase. (A) Glutaraldehyde cross-linking. A 2 µg aliquot of the enzyme was incubated with 0, 0.0015, 0.003, 0.006 and 0.0125% glutaraldehyde (lanes 1–5) and the sample was analyzed by SDS–PAGE. Lane M refers to the protein molecular weight markers as indicated. The monomer and dimer positions of the REase are indicated. (B and C) Gel filtration chromatographic profile. The REase was applied onto a Superdex-200 column and analyzed as described in Materials and Methods. The different molecular weight markers were run separately to obtain a calibration curve (B). The fractions were assayed for the presence of REase activity and plotted against elution volume (C).