Figure 2.
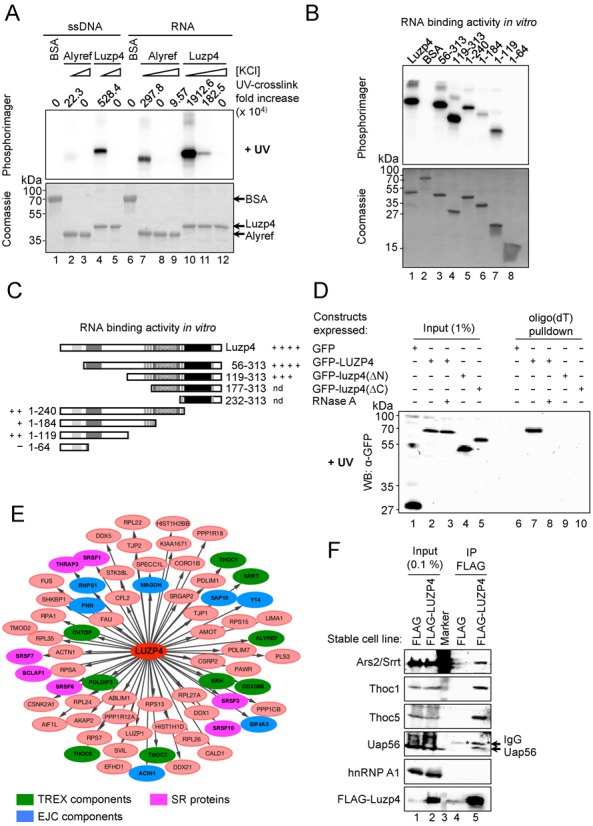
Luzp4 is an RNA binding protein that associates with TREX subunits. (A) UV-crosslinking assays using recombinant Alyref, Luzp4 or BSA control and 32P-labelled single-stranded RNA or DNA oligonucleotides. Lanes 2, 4, 7, 10 had 100 mM KCl, lanes 8, 11 had 300 mM KCl and lanes 3, 5, 9, 12 had 500 mM KCl in the reactions. The upper panel shows the phosphoimage of the same gel which is Coomassie stained in the lower panel. The quantification shows the increase of crosslinking relative to the relevant BSA-containing reaction (ssDNA or ssRNA). Results where UV crosslinking was omitted were exposed and acquired at the same time and are presented in Supplementary Figure S3A. (B) UV crosslinking of 32P-labelled RNA oligonucleotide with the indicated purified truncations of Luzp4 in 100 mM NaCl. The upper panels show the phosphoimage of the Coomassie-stained gels shown in the lower panels. (C) Schematic of the Luzp4 truncations used to map the RNA binding activity and summary of the RNA binding activity for truncations measured using UV crosslinking. nd: not determined because the constructs are insoluble and precipitated repeatedly during refolding step following purification. (D) Luzp4 binds poly(A)+-RNAs in vivo. The indicated GFP fusions of Luzp4 were analysed for binding to poly(A)+-RNAs in vivo using UV crosslinking. Proteins were visualized by western blot with anti-GFP antibody. Results where UV crosslinking was omitted were exposed and acquired at the same time and are presented in Supplementary Figure S3B. (E) Summary of the interacting proteins identified using mass spectrometry following IP of FLAG-Luzp4 stably expressed in Flp-In™ T-REx™ 293 cells (see Materials and Methods). (F) Validation of the mass spectrometry samples. Proteins co-immunoprecipitating with FLAG or FLAG-Luzp4 were detected using western blotting with antibodies to the indicated proteins and FLAG-Luzp4 was detected using FLAG antibody.
