Figure 5.
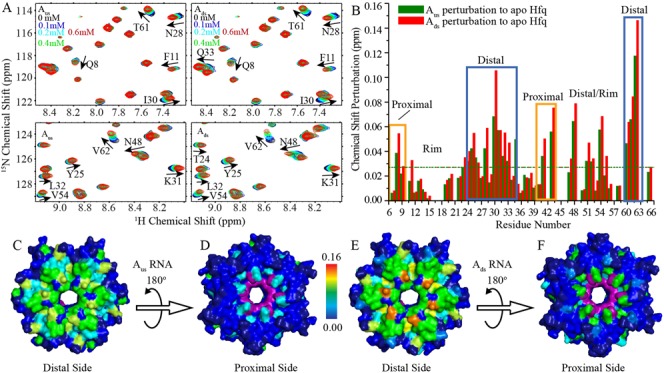
NMR chemical shift perturbations of Hfq by Aus and Ads. Hfq65 R16A/R17A protein (0.1 mM) were titrated with Aus and Ads, respectively. (A) Selected regions of 1H-15N HSQC spectra of Hfq upon Aus (left) and Ads (right) titration. (B) Chemical shift differences between the first and last titration points for Aus and Ads are presented as green and red column bars, respectively. (C, E) Both Aus and Ads binding to Hfq result in prominent chemical shift perturbations on the distal side of Hfq. (D, F) Residues Q8, Q41 and V43 on the proximal side of Hfq are also perturbed by Aus and Ads titration. Hfq is colored according to chemical shift changes using a blue to red gradient. The resonances of F42 and H57, which disappeared upon Aus and Ads titration, are colored purple. Unassigned residues are colored dark blue.
