Figure 11. Nickel-catalyzed Heck reactions.
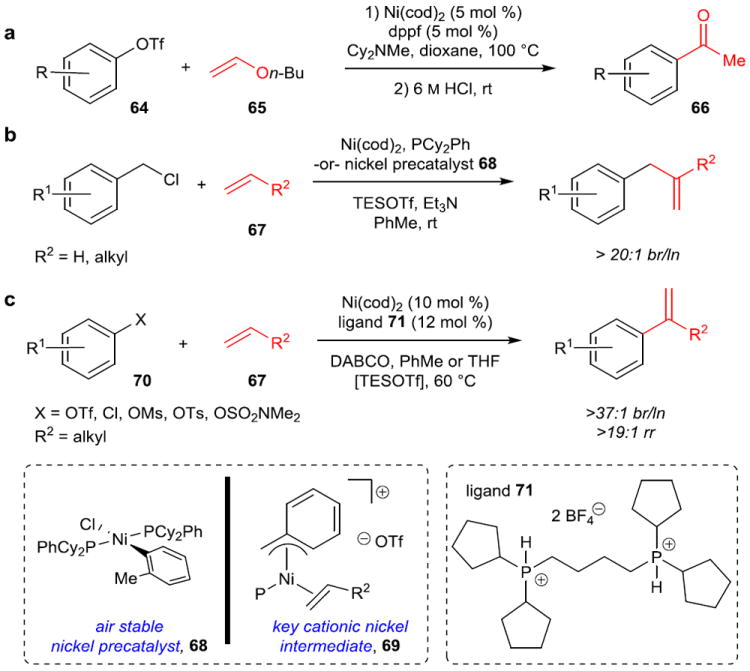
a, Coupling of aryl triflates (64) with electron-rich enol ethers (65) to obtain high selectivity for branched products, which upon acidic hydrolysis form ketones. Computational work supports a cationic Heck pathway with catalyst regeneration as turnover-limiting. b, The first Heck reaction highly selective for branched products with electronically unbiased (aliphatic) and non-chelating alkenes (67). Again, it was proposed to proceed through a cationic Ni species (69) to give high regioselectivity, and an air stable precatalyst (68) was developed to eliminate the need for air-free technique. c, Branch-selective Heck reaction for aryl electrophiles (70) with aliphatic olefins (67). Bidentate ligand 71 was key to both reactivity of aryl electrophiles and suppression of undesired isomerization. Aryl chlorides and other phenol-derived electrophiles can be utilized with the use of TESOTf, which is proposed to perform a counterion exchange in order to enter the cationic Heck pathway. TESOTf, triethylsilyl trifluoromethylsulfonate; DABCO, 1,4-diazabicyclo[2.2.2]octane.
