Figure 12. Prototypical reductive coupling reactions and use of new reducing agents.
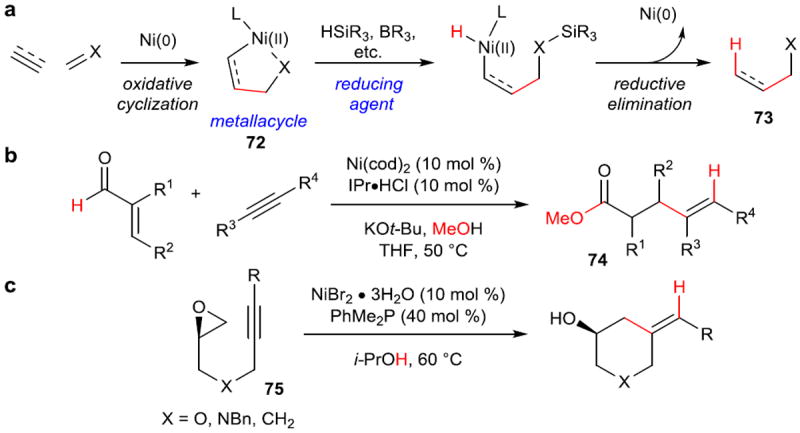
a, Standard reductive coupling reaction. Oxidative cyclization of two π-components forms a nickallacycle (72), which upon formation of a Ni–H bond with a reducing agent, undergoes reductive elimination to form a new C–C σ-bond and a new C–H bond overall (73). b, Use of methanol as a mild reducing agent via the intermediacy of a hemiacetal. c, Use of isopropanol as a mild, external reducing agent, allowing for the use of air-stable Ni(II) salts as precatalysts. IPr, 1,3-bis(2,6-diisopropylphenyl)-1,3-dihydro-2H-imidazol-2-ylidene.
