Figure 13. Regiocontrol in reductive coupling reactions: two strategies.
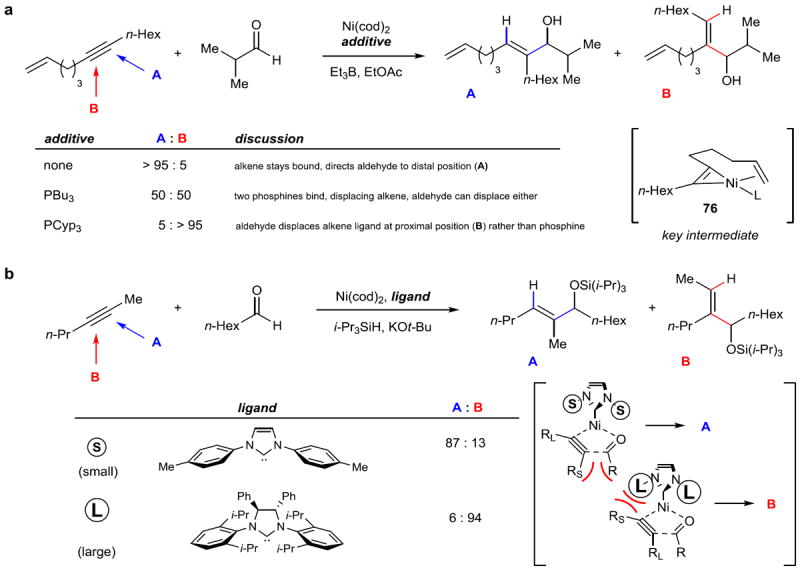
a, A tethered alkene can be used to control regioselectivity in coupling an alkyne with an aldehyde. The alkyne oxidatively adds to Ni to produce nickallacyclopropene 76. Then, by selective displacement by added phosphine ligands, or with no additive, the binding and orientation of the aldehyde is controlled to produce either linear (A) or branched (B) products. b, The steric profile of carbene ligands can be used to control the regioselectivity of an alkyne–aldehyde reductive coupling. Computational work suggests that unfavorable steric interactions between the groups on the alkyne with either the group on the aldehyde or the groups on the ligand dictate the orientation of the forming five-membered nickallacycle intermediate (shown in brackets). EtOAc, ethyl acetate; Cyp, cyclopentyl.
