Figure 2. Halogen alternatives used in cross-coupling reactions.
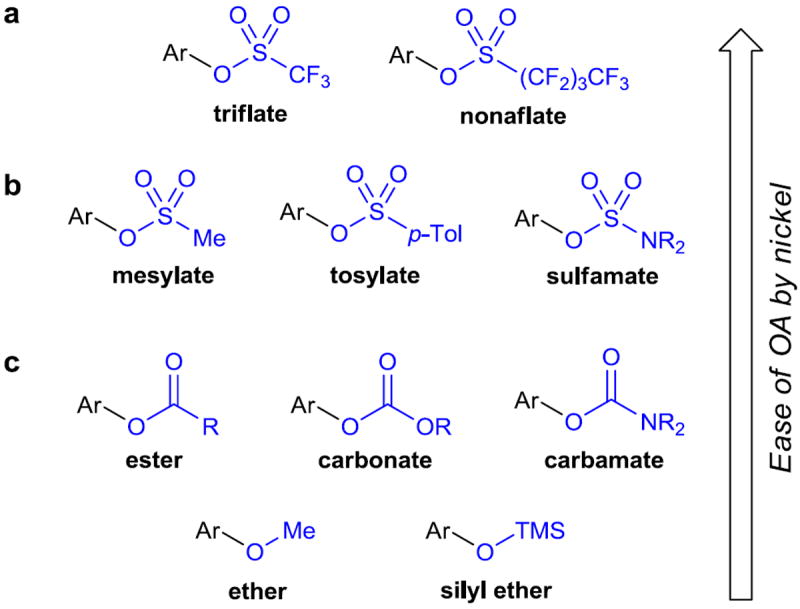
a, Aryl triflates have long been used as replacements for halogens in cross-coupling reactions. Aryl nonaflates were developed later to address some of the issues encountered when working with aryl triflates, but their use is less widespread. b, The use of aryl mesylates, tosylates, and sulfamates presents many advantages over triflates and related fluorinated sulfonates due to their increased stability. c, Like sulfonate derivatives, the use of carboxylic esters, carbonates, carbamates, ethers, and silyl ethers can be advantageous in many situations. Ar, aryl; p-Tol, para-tolyl (4-methylphenyl); TMS, trimethylsilyl.
