Figure 7. Proposed mechanisms for Csp3–Csp3 cross-coupling.
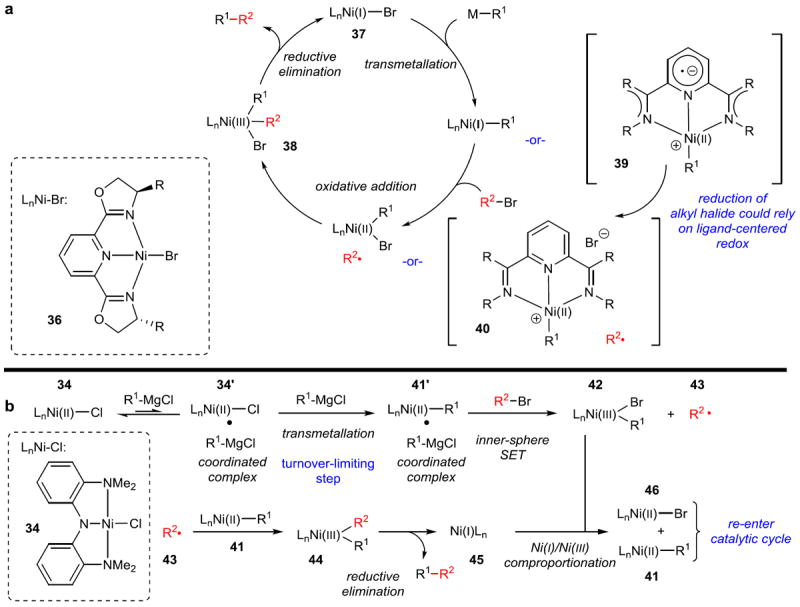
a, Mechanism proposed for Fu-type cross-coupling reactions in which ligands are typically PyBOX or similar as shown (36). A Ni(I) complex (37) undergoes transmetallation, then a radical oxidative addition of the electrophile, to eventually form a Ni(III) complex (38), which can reductively eliminate the coupled product. Nickel complexes with redox active ligands have been shown to be perhaps better thought of as the species in brackets (39, 40), in which the oxidative addition to the electrophile proceeds through ligand-centered rather than metal-centered redox. Ni(II) compounds are used as precatalysts, allowing for reactions to be set up outside of the glovebox. Reduction to Ni(I) presumably occurs prior to the beginning of the catalytic cycle via reduction of Ni(II) to Ni(0) (transmetallation/reductive elimination), then comproportionation of Ni(0)/Ni(II). b, Mechanism proposed for the Csp3–Csp3 Kumada cross-coupling with Ni-pincer 34. Extensive mechanistic studies have shown that a more complex bimetallic oxidative addition is operative, in which a Ni(II) complex bound to an equivalent of Grignard reagent (34’) is the active complex for the turnover-limiting transmetallation. SET, single electron transfer.
