Figure 9. Reductive cross-coupling reactions.
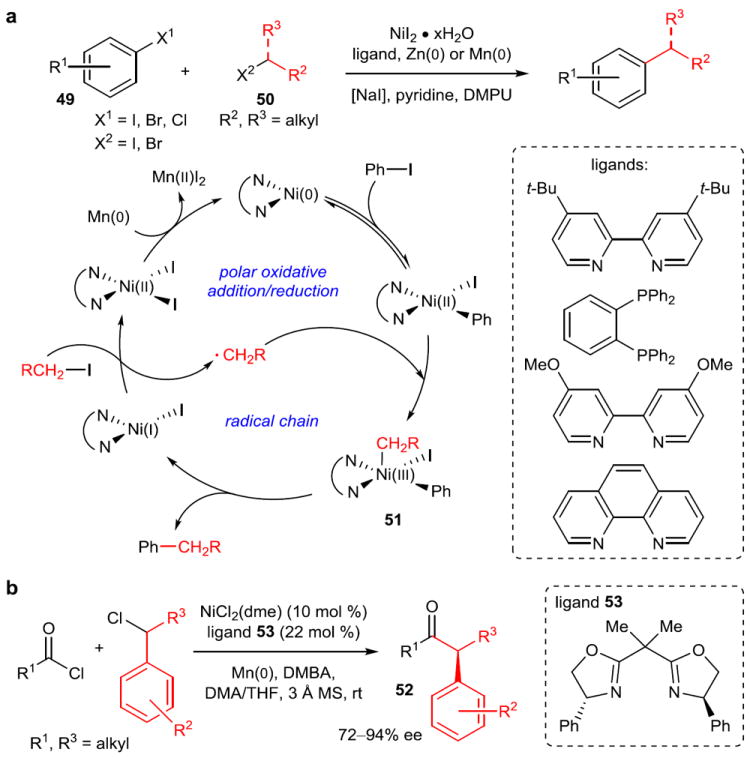
a, The reductive cross-coupling reaction of an aryl halide (49) with an alkyl halide (50) without the intermediacy of an organozinc or organomanganese species. Extensive mechanistic studies have suggested that this method combines both polar (aryl halide) and radical chain (alkyl halide) formal oxidative addition mechanisms. Because the oxidation state of nickel is matched to each electrophile, homodimerization is suppressed. For details on possible methods of radical chain initiation, see reference 77. b, First asymmetric acyl reductive cross-coupling. High enantioselectivities are obtained with bisoxazoline ligands such as 53. DMPU, 1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone; DMBA, 2,6-dimethylbenzoic acid; MS, molecular sieve.
