Table 1.
Calculated reaction energies (ΔE=∑Eproducts−∑Ereactants), independent equilibrium constants (300 K) and concentrations for Schottky disorder in CH3NH3PbI3. For partial disorder the chemical potentials are taken to be pinned to the formation of PbI2 and CH3NH3I, respectively. The values of KC (%) are normalized to the site fraction of vacancies, and n refers to the vacancy defect concentration
| Reaction | ΔHS [eV per defect] | KC | n [cm−3] |
|---|---|---|---|
nil→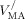 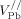 +3 +3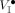 +MAPbI3 +MAPbI3
|
0.14 | 0.41 | 2×1019 |
nil→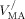 + +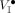 +MAI +MAI |
0.08 | 3.82 | 2×1020 |
nil→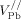 +2 +2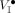 +PbI2 +PbI2 |
0.22 | 0.02 | 8×1017 |
