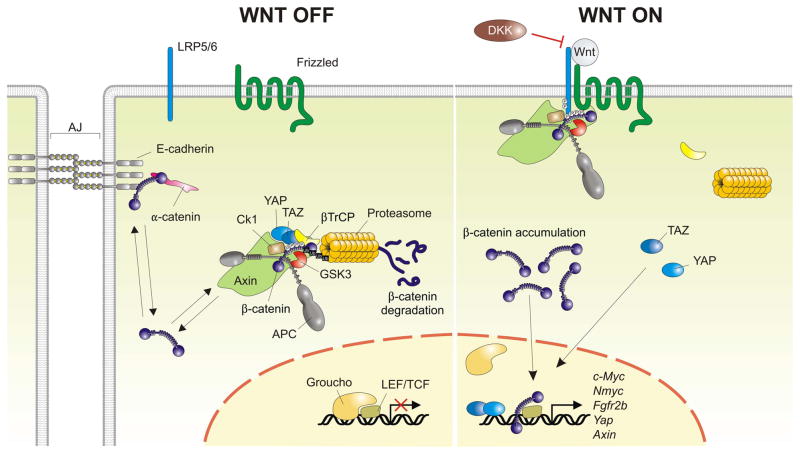
Fig. 3. The canonical Wnt signaling pathway.
In the absence of Wnt ligands (Wnt OFF), cytosolic β-catenin that does not participate in adherens junction (AJ) stabilization is captured by the destruction complex, where it is first phosphorylated, and then ubiquitinated by recruiting β-TrCP followed by its proteasome-mediated degradation. According to the latest model, binding of Wnt ligands to their Lrp5/6-Frizzled receptor (Wnt ON) recruits the intact destruction complex to the activated receptor where it is still able to capture and phosphorylate β-catenin. Binding of Axin to activated LRP dislodges the Hippo co-transcriptonal effectors YAP/TAZ from the destruction complex, rendering β-catenin invisible to β-TrCP. As a result, β-catenin can no longer be ubiquitinated and newly synthesized β-catenin accumulates in the cytoplasm and translocates to the nucleus where it replaces the repressor Groucho and binds to LEF/TCF trancription factors to induce Wnt target genes. Note that once dislodged from the destruction complex, YAP/TAZ can translocate to the nucleus as well where they interact with TEAD transcription factors to initiate gene expression. YAP/TAZ/TEAD-mediated gene expression is therefore an integral part of the Wnt response. DKK proteins inhibit Wnt signaling by preventing the interaction of Wnt ligands with LRP5/6.