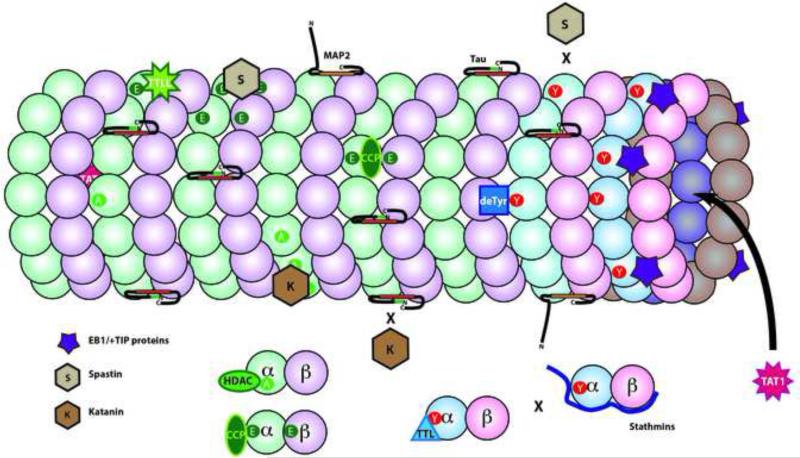
Figure 4. Microtubule Associated Proteins and Posttranslational Modifications of Tubulins.
One function of tubulin modifications is to affect the interaction of other proteins and enzymes. In complex with EB1, some +TIP binding proteins (purple 5 point star) bind selectively to tyrosinated tubulin. As a result, these +TIP proteins are enriched on the plus end of dynamic microtubules, which are rich in newly polymerized tyrosinated α-tubulin. Other proteins interact with particular tubulin isotypes like a subset of α-tubulins (deTyr for detyrosination) or to the C-termini of both α- and β-tubulins (TTLLs for polyglutamylation or polyglycylation). Tubulin acetylases (TAT1) are unique in binding to the interior of the microtubule lumen. Deacetylases (HDAC) or Tubulin Tyrosine Ligase (TTL) bind only to tubulin dimers. Stathmins bind to free tubulins as well, but partially overlap and compete with tubulin binding to TTL. The microtubule severing proteins, katanin (K) and spastin (S) are directed to particular microtubule domains by a combination of tubulin modifications and microtubule associated proteins. Katanin preferentially binds and severs acetylated domains of a microtubule, but katanin binding is inhibited by the presence of tau. In contrast, spastin preferentially binds and severs in polyglutamylated domains, but does not sever microtubules enriched in tyrosinated tubulin.