Figure 1.
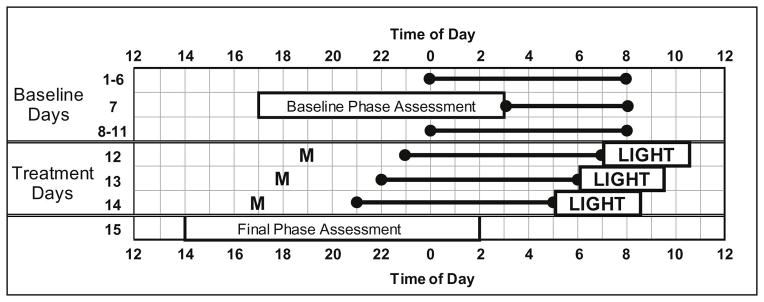
Protocol Diagram. The 2-week protocol included Baseline Days (1–11) and Treatment Days (12–14). Days 12, 13, and 14 are also referred to as Treatment Days 1, 2, and 3 respectively in the text. The horizontal lines anchored with circles illustrate scheduled sleep times. A participant with a baseline bedtime of midnight (0) and baseline wake-up time of 08:00 is shown as an example. Baseline sleep schedules always allowed for 8 hours of time in bed, but bed and wake times varied among participants as they were based on self-reported sleep times before beginning the study. The sleep schedule was gradually advanced on days 12, 13 and 14. Participants ingested 0.5 mg melatonin (M) 5 hours before their baseline bedtime on the first treatment day and then 1 hour earlier on each subsequent treatment day. Bright light (~ 5000 lux) from a single light box was administered within 5 minutes of waking on each of the 3 treatment days. On Days 7 and 15, participants completed a circadian phase assessment to determine the dim light melatonin onset (DLMO).
