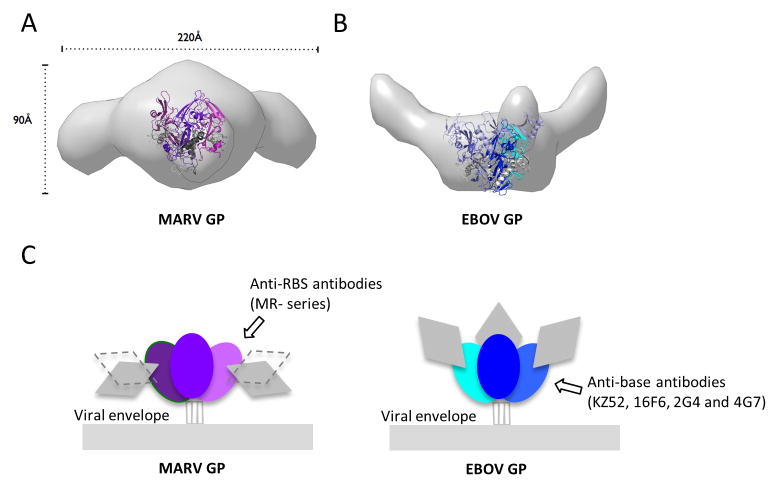
Figure 5. MARV and EBOV present different surfaces for antibody recognition.
(A and B) Molecular envelopes of mucin-containing MARV and EBOV GP ectodomains determined by SAXS. Rendered Gaussian distributions of molecular envelopes are illustrated in light gray, with ribbon models of the crystallized MARV GPcl and EBOV GPΔmuc trimers to scale and overlaid for comparison. The trimers are illustrated as ribbons. Note that the glycan cap was removed from MARV GP used in crystallization in order to improve diffraction, but was contained in the complete MARV GP used for SAXS. The glycan cap did not inhibit diffraction of EBOV GP and is included in the EBOV GP crystal structure. MARV GPcl is colored in purple (GP1) and gray (GP2). EBOV GPΔmuc is colored blue (GP1), white blue (GP1 glycan cap) and gray (GP2). MARV GP is drawn in two possible orientations because definitive placement of polypeptide is challenging at this resolution. In either orientation however, the mucin-like domains of MARV project sideways, equatorially or downwards from the core of GP. In MARV, the mucin-like domain is attached to both GP1 and GP2. By contrast, in EBOV, the mucin-like domain is attached solely to GP1, there is no anchor at the base. Both these SAXS experiments and previous electron tomography (Tran et al., 2014) agree on the upward projection of the mucin-like domains in EBOV. See also Figure S5. (C) Differing positions of the mucin-like domains between MARV and EBOV may lead to elicitation of different types of antibodies. The lower position and GP2 anchor of the mucin-like domain of MARV may better mask the base of GP, but expose its upper surfaces, allowing antibodies like mAb MR78 to be elicited. The upwards projection of the EBOV mucin-like domain and absence of any GP2 anchor, appear to better mask upper surfaces, but expose the base, allowing antibodies such as KZ52 (Lee et al., 2008), 2G4, 4G7 (Murin et al., 2014) and 16F6 (directed against Sudan ebolavirus (Dias et al., 2011; Bale et al., 2012)) to be elicited.