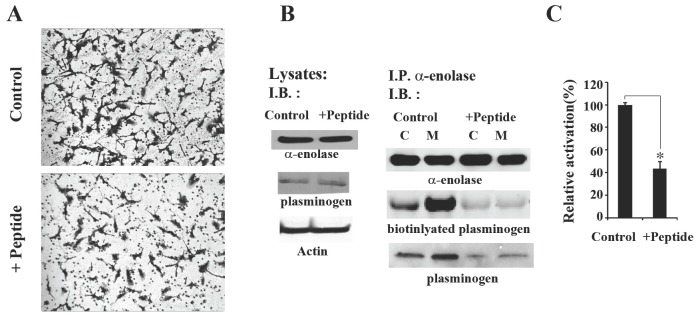
Fig. 3. Seahorse peptide inhibits invasive migration of HT1080 cells by decreasing interaction between α-enolase and plasminogen. (A) Matrigel invasion assays were performed with HT1080 cells incubated with 0.1 M peptide for 48 h. (B) Peptide treatment decreases the association of α-enolase and plasminogen. HT1080 cells were incubated with 0.1 M control peptide or seahorse peptide for 48 h and then the cell lysates were analyzed for α-enolase, plasminogen, and a-actin. Peptide treatment did not affect the expression levels of α-enolase and plasminogen in HT1080 cells. Additionally, the cell lysates were subfractionated into 100,000 g pellet (membrane) or supernatant (cytosol) fractions. Total 200 μg protein from each fraction was immunoprecipitated with an anti-α-enolase antibody, and immunoprecipitae were separated by SDS-PAGE and transferred to PVDF. The levels of protein in each immunoprecipitate were determined by immunoblotting with antibodies against α-enolase and plasminogen. Overlay assay with 0.1 μg biotinylated plasminogen was also performed to confirm to detectable binding between α-enolase and plasminogen. Specific protein interaction was shown as streptavidin signal. Results are representative of three separate experiments. (C) Peptide treatment attenuates activation of plasminogen in cell membrane. Cells incubated with 0.1 M control peptide or seahorse peptide were lysed, and then fractionated into membrane fraction. 20 μg of membrane fraction was added to assay for plasminogen activity. The results are expressed as the average of three different experiments. *P < 0.05 compared to controls peptide treatment.