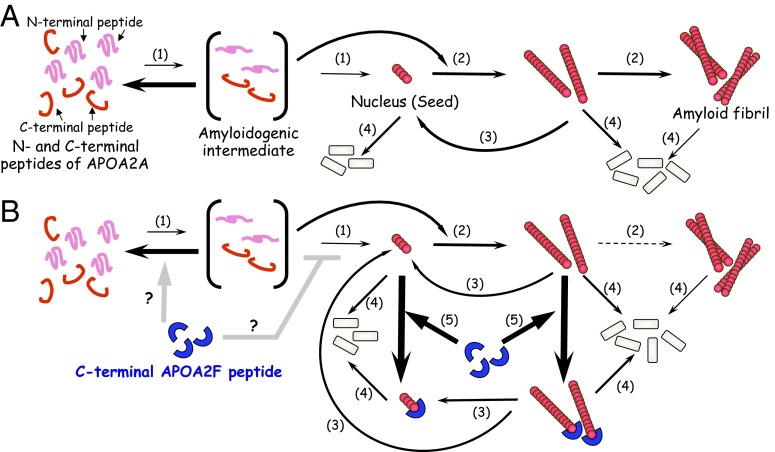
Fig. 9.
Models showing amyloid fibril formation by apoA-II protein and the selective inhibition by the C-terminal APOA2F peptide. (A) Amyloid fibril formation of apoA-II peptides in vitro (23). N- and C-terminal APOA2A peptides change their secondary structures under acidic conditions (pH 2.5) with amyloidogenic conformation (intermediate) and polymerize into a nucleus (seed) (step 1). Once a nucleus is formed, or new nuclei (seeds) are made by fragmentation of amyloid fibrils, further extension of amyloidogenic peptides occurs (steps 2 and 3). Amyloid fibrils are degraded in the course of time, except for the supply of combination of N- and C-terminal amyloidogenic peptides (step 4). (B) Hypothetical mechanism by which the C-terminal APOA2F peptide inhibits amyloid fibril formation. The C-terminal APOA2F peptide strongly inhibits the extension phase of amyloidogenic peptides by capping possible active ends of seeds (step 5). As a result, amyloidogenic APOA2A peptides cannot polymerize into amyloid fibrils (step 2).