Abstract
This study was to investigate the role of Fas in the development of Cisplatin-resistant ovarian cancer. On the cellular level, Fas expression was significantly reduced in Cisplatin resistant A2780 (A2780/CP) cells compared with A2780 cells. Fas silence with siRNA would promote tumor cell lines proliferation, facilitate tumor cell cycle transition of G1/S, prevent cell apoptosis, and promote cell migration. Expression of drug resistance gene was negatively correlated to Fas. In nude mice metastasis model of human ovarian carcinoma by subcutaneous transplantation, after Ad-Fas injected intratumorly, we found that upregulation of Fas could inhibit transplantation tumor tissue growth and reduce the expression of drug resistance gene. Our results indicated that upregulation of Fas in epithelial ovarian cancer reversed the development of resistance to Cisplatin. In conclusion, our findings suggested that Fas might act as a promising therapeutic target for improvement of the sensibility to Cisplatin in ovarian cancer. [BMB Reports 2015; 48(1): 30-35]
Keywords: A2780/CP cell, Cisplatin-resistance, Epithelial ovarian cancer, Fas, Oncotherapy
INTRODUCTION
Ovarian cancer is common gynecologic malignant tumor around the world, and is one of the leading mortality causes in women (1). Platinum-based chemotherapeutic strategies are broadly required for patients with ovarian cancer, but Cisplatin-resistance has been a major barrier to the successful ovarian cancer treatment (2)). Resistance to platinum has been reported to be associated with several mechanisms, including increased drug efflux (3), drug inactivation (4), alterations in drug target (5), processing of drug-induced damage (6) and evasion of apoptosis (7). However, the direct evidence of apoptosis evasion in ovarian cancer has not been clearly found out.
Fas is a prototypical death receptor consisting of a N-terminal region containing three cysteine-rich domains (CRDs), with ligand binding occurring predominantly at the second and third CRDs, a transmembrane domain and an intracellular region containing an less than 80 aminoacid domain called the “death domain” (DD) (8). It is ubiquitously expressed in the body, but is particularly abundant in liver, heart, brain, and colon tissues, and in activated mature lymphocytes (9). Fas was reported to be involved in many physiological processes such as proliferation, apoptosis, migration etc. In these years Fas has attracted more and more attention in cancer progression. It was reported that upregulation or hyperactivation of fatty acid synthase (FAS) has recently been found in most ovarian cancer and in most human solid tumors, where it is highly associated with high aggressiveness and poor patient survival (10-12). Inhibition of FAS activity is selectively cytotoxic to human cancer cells in vitro and in vivo including human ovarian cancer xenografts (13). However, the mechanisms linking the inhibition of Fas activity to cancer cell proliferation, apoptosis and migration need to be researched intensively. In this study, we also focused on the role of Fas regulation in the treatment of Cisplatin-resistant ovarian cancer.
RESULTS
Down regulation of Fas is associated with development of Cisplatin resistance
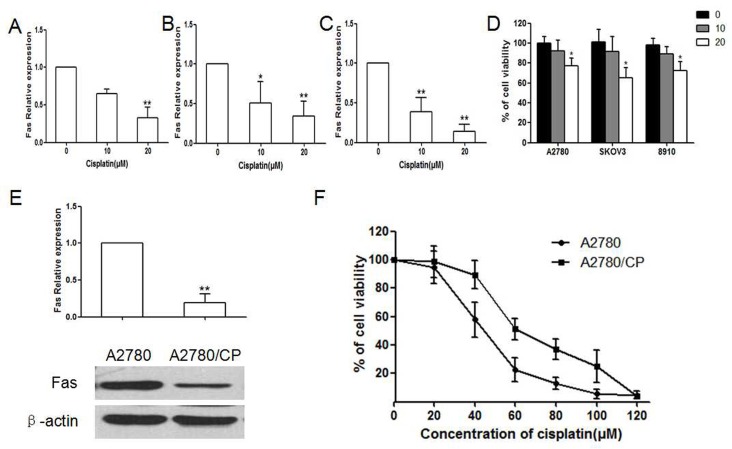
Western blot results showed that after treatment with various concentration of Cisplatin, the expression of Fas decreased in a concentration-dependent manner in A2780, SKOV3 and ES2 cells (P < 0.05, Fig. 1A-C). And, Cell viabilities of A2780, SKOV3 and 8910 were significantly lower than vehicle control after treated with 20 μM Cisplatin (P < 0.05, Fig. 1D). Fas expression was remarkably decreased in Cisplatin-resistant A2780 cells when compared with parent A2780 cells (P < 0.01, Fig. 1E). Besides, the half maximum inhibitory concentration of Cisplatin to A2780 was significantly lower than A2780/CP (IC50:43.56 versus 65.75 μM, Fig. 1F). These results indicated that decreased Fas might be associated with Cisplatin-resistance ovarian cancer.
Fig. 1. Effect of Cisplatin on expression of Fas and cell viability in sensitive and resistant ovarian cancer cell lines. (A-C) Expression of Fas in sensitive ovarian cancer cell lines A2780, SKOV3 and 8910 after treated with Cisplatin in in 0, 10, 20 μM (*P < 0.05, **P < 0.01, compared with 0). (D) Cell viability of A2780, SKOV3 and 8910 after treated with Cisplatin in 0, 10, 20 μM (*P < 0.05, compared with 0). (E) Expression of Fas in epithelial ovarian cancer cell line A2780 and A2780/CP cells after treated with Cisplatin (0, 10, 20 μM) for 48h. (**P < 0.01, compared with A2780). (F) Comparation of cell viability of A2780 and A2780/CP cells treated with Cisplatin in different concentration.
Fas silence promotes cell proliferation and cell cycle transition
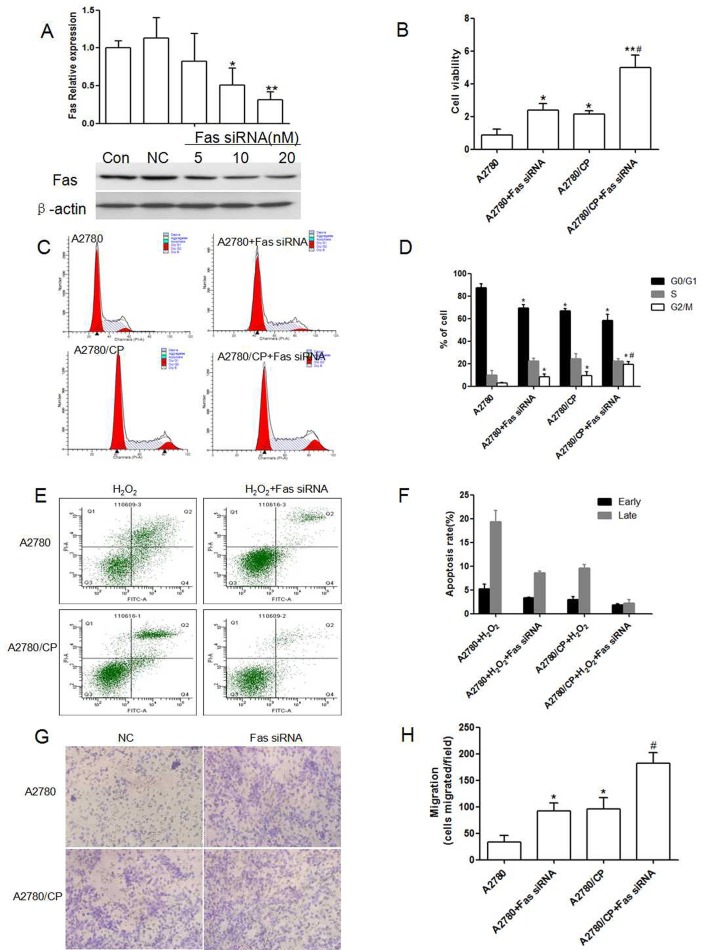
Small interfere RNA technology was taken to investigate the silence effect of Fas. We screened the effective Fas siRNA concentration in A2780, and the interfere efficiency reached to 73% (Fig. 2A). Next, we observed the effect of Fas siRNA on cell proliferation, and cell cycle transition. Fas was proven to be involved in cell proliferation, our results also demonstrated that Fas silence could promote A2780 and A2780/CP cells proliferation, and this effect was enlarged in A2780/CP cells (Fig. 2B). As shown in Fig. 2C, Fas silence decreased the percentage of cells in the G0/G1 phase from 87.36 ± 3.26% to 69.15 ±3.41% and enhanced the percentage of cells in the S phase from 9.62 ± 4.13% to 19.32±2.56% (P < 0.05; Fig. 2D). And this effect was enhanced in A2780/CP cells. Our data indicates that Fas silence promoted cell cycle transition from the G0/G1 phase to S phase in A2780 and thus promoted cell proliferation, and Cisplatin resistance had much significant effect on cell cycle transition.
Fig. 2. Effect of Fas silence on proliferation, cell cycle, apoptosis and migration of A2780 and A2780/CP cells. (A) Screen of Fas siRNA concentration in A2780 cells (*P < 0.05, **P < 0.01, compared with negtive control). (B) Effect of Fas silence on proliferation of A2780 and A2780/CP cells (*P < 0.05, **P < 0.01, compared with A2780, #P < 0.05, compared with A2780 + Fas siRNA). (C) (D) Effect of Fas silence on cell cycle transition of A2780 and A2780/CP cells (*P < 0.05, **P < 0.01, compared with A2780, #P < 0.05, compared with A2780+Fas siRNA). (E, F) Effect of Fas silence on apoptosis of A2780 and A2780/CP cells. (G, H) Effect of Fas silence on cell migration of A2780 and A2780/CP cells (*P < 0.05, **P < 0.01, compared with A2780, #P < 0.05, compared with A2780 + Fas siRNA).
Cisplatin-resistant A2780 cells was sensitive to Fas silence induced apoptosis
Effects of Fas silence on A2780 apoptosis was analyzed by Annexin V-FITC/PI flow cytomety. In Fig. 2, transfection with Fas siRNA could inhibit H2O2 induced apoptosis in A2780 an apoptotic rate of 24.36 ± 3.25%, which was much lower than H2O2 treated A2780 cells (P < 0.01; Fig. 2E), and transfection with Fas silence on Cisplatin-resistant A2780 cells induced an apoptotic rate of 4.54 ± 1.67%, which was also lower than H2O2 treated A2780/CP cells (P > 0.05; Fig. 2F). This result showed that Fas silence induced apoptosis was enhanced in A2780/CP cells.
Cisplatin promotes Fas silence induced cell migration and invasion
To detect whether knockdown of Fas affects the migration of A2780 and A2780/CP cells, transwell migration assay was performed to assess the proportion of cells which migrated through polycarbonate membranes after transfection for 48h. We found that Fas knockdown increased the number of invaded cells accounting for 52.2% rise in A2780 cells (Fig. 2G, H, P < 0.01), besides, A2780/CP cells also increased the number of invaded cells, Fas silence definitely boostered this invasion. So Fas silence promotes the ability of migration and invasion of A2780 cells, while Cisplatin resistance could promote this ability of A2780 cells.
Fas silence influences Cisplatin induced the expression of MDR1, MRP2 and LRP
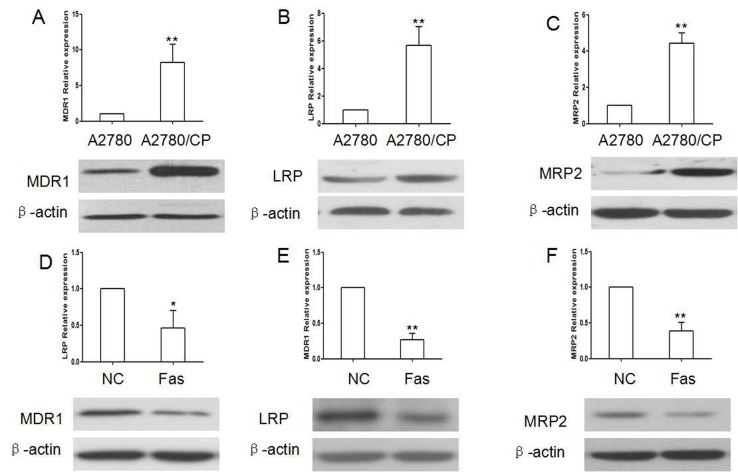
To further investigate the mechanism that how deos Fas impact Cisplatin resistance, we observed the effect of Fas silence on expression of drug-resistance gene MDR1, MRP2 and LRP. The results showed that A2780/CP cells had higher expression of MDR1, MRP2 and LRP than A2780 cells. Fas silence could reduce the expression of MDR1, MRP2 and LRP in A2780 cells, while Fas overexpression could reverse their expression in A2780/CP cells (Fig. 3).
Fig. 3. Drug-resistant genes were regulated by Fas. (A-C) Drug-resistant genes were remarkably up regulated in A2780/CP cells. (D-F) Fas overexpression could reduce the expression of MDR1, LRP and MRP2 in A2780/CP cells (*P < 0.05, **P < 0.01, compared with).
Re-expression of Fas could reverse resistance in ovarian cancer in vivo
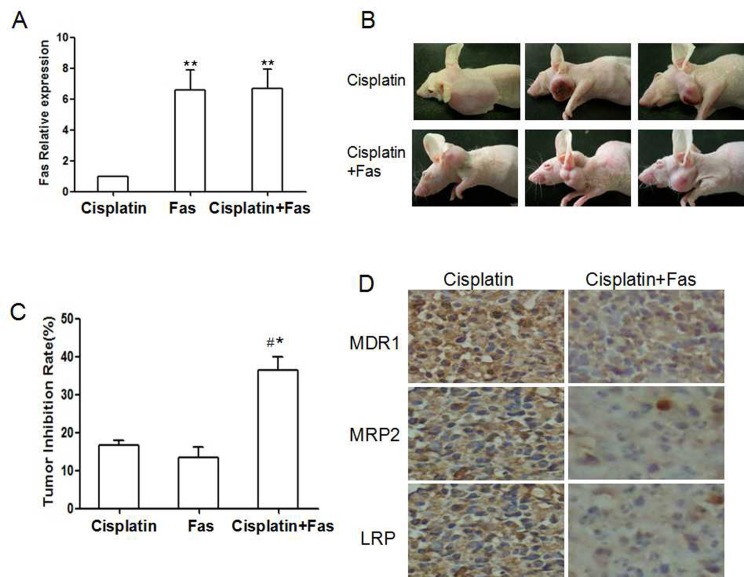
To study whether overexpression of Fas is effective in inhibiting drug-resistant ovarian cancer in vivo, we established transplantation tumor model by subcutaneous injection with A2780 cells in nude mice. 12 days later, the nude mice were treated with Cisplatin alone or combined with Fas over expression vector. Fas expression was remarkably increased in tumor in combination group, suggesting that Ad-Fas was effective in vivo (Fig. 4A, P < 0.01). Then we found that the transplantation tumors in the combination group tended to grow more slowly than those in Cisplatin alone group. After 35 days in combination group, tumor size was 1842.16±325.64 mm3, smaller than 2957.13±29.16 mm3 in those given Cisplatin alone (Fig. 4B, C, P < 0.01). In line with results of our experiments in vitro, immunohistochemistry staining of tumor sections revealed a dramatic decrease in the expression of MDR1, MRP2 and LRP in tumors received combination therapy (Fig. 4D).
Fig. 4. Overexpression of Fas could reverse the chemoresistance in vivo. (A) Western blot analysis of Fas expression in nude mice treated with Cisplatin, Ad-Fas and Ad-Fas combination with Cisplatin. (B) Representative images of transplanted tumor nude mice treated with Cisplatin and Ad-Fas combination with Cisplatin. (C) Tumor volumes of nude mice treated with Cisplatin, Ad-Fas and Ad-Fas combination with Cisplatin. *P < 0.05, **P < 0.01, compared with treatment of Cisplatin; #P < 0.05, compared with treatment of Ad-Fas. The arrows indicated treatment. (D) Representative results of immunohistochemical staining of MDR1, MRP2 and LRP in the tumor sections of nude mice (magnification 400X).
DISCUSSION
Many recent studies have proven that Fas played a critical role in pathogenesis of ovarian cancer, some evidences suggested that Fas might be connected to Cisplatin-resistance of cancer therapy (14). In our present study, we confirmed that expression of Fas decreased in Cisplatin-resistant A2780 cells, which indicated Fas might played the protective role in Cisplatin resistance of ovarian cancer. Then we designed serious of experiments to explore the involving mechanism. Consistent with other reports, Fas inhibition promoted A2780/CP cells proliferation and migration, but inhibit A2780/CP cells apoptosis, which were consistent with results in A2780 cells and brought adverse effect for Cisplatin-resistance tumor therapy. Except for these in vitro evidences, we also got direct data from nude mice of transplantated-tumor model. We found that artificially elevated expression of Fas, played a good inhibitory effect in tumor treatment, such as minimize tumor size and reduce expression of drug resistance gene.
Fas was reported to up regulate in various of human solid tumor including breast, stomach, lung, ovary and so on (15-18), which suggested the functional role of Fas in the progression of malignant cancer. Inhibition of Fas activity could promote tumor cell growth by arresting cell cycle transition and inhibit apoptosis by affecting Bad pathway in ovarian cancer (19). So Fas was considered as a potential target for anticancer therapy. But as the drug-resistant problem in tumor therapy is becoming more and more serious, especially for platinum chemotherapy, whether Fas regulation could be useful in Cisplatin-resistant ovarian cancer deserved to be investigated furtherly.
Cell abnormal proliferation and apoptosis contributed to pathogenesis of tumor. Cancer growth was controlled by the balance between cell proliferation and apoptosis. According to this point, regulation of cell proliferation and apoptosis could become an effective pathway to cancer therapy. Consistent with previous reports, we confirmed that Fas inhibition really increased cell proliferation and prevented apoptosis in A2780 cells, and Fas inhibition could also promote proliferation and inhibit apoptosis of Cisplatin-resistant A2780 cells. Anyway, cell proliferation had been closely related to cell cycle. If cell cycle arrest, cell growth was bound to be affected. The results showed that Fas inhibition promoted transition of G0/G1 to G2/M.
Cell migration and invasion was important process involved in pathological processes (20). The high mortality rate of ovarian cancer patients was attributed to the highly invasive characteristic of the disease (21). Besides, malignant ovarian epithelial cells primarily spread to adjacent organs by local invasion. So inhibition of cell migration could be an important way to control ovarian cancer. In our study, Fas inhibition promoted cell migration in A2780 by transwell assay, and this ability was enlarged in A2780/CP cells.
Our studies demonstrated that down regulation of Fas was involved in Cisplatin resistance development in ovarian cancer cell lines. The in vitro study proved that Fas played an protective role in cancer development. There were reports that Fas gene transduction could reverse the multidrug resistance of human drug resistant SCLC cell H446/CDDP, for which the enhanced cell sensitivity to apoptosis and decreased expression of GST-π and ERCC1 might be responsible (14). That means up-regulation of Fas reverse Cisplatin resistance of human small cell lung cancer cell. Our data also indicated that treatment with Fas overexpression combined with Cisplatin could significantly minimize the growth of tumors in vivo, suggesting higher Fas level might be required for more effective tumor growth inhibition. Fas regulation might be the target of solving Cisplatin resistance in ovarian cancer therapy.
MATERIALS AND METHODS
Materials and Methods are available as supplementary data on BMB Reports online.
Acknowledgments
This work was supported by National Natural Science Foundation of China (No.81260377).
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics. CA Cancer J Clin. (2007);57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Broxterman HJ, Gotink KJ, Verheul HM. Understanding the causes of multidrug resistance in cancer: a comparison of doxorubicin and sunitinib. Drug Resist Updat. (2009);12:114–126. doi: 10.1016/j.drup.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Song IS, Savaraj N, Siddik ZH, et al. Role of human copper transporter Ctr1 in the transport of platinum-based antitumor agents in Cisplatin-sensitive and Cisplatin-resistant cells. Mol Cancer Ther. (2004);3:1543–1549. [PubMed] [Google Scholar]
- 4.Stefansson OA, Villanueva A, Vidal A, Martí L, Esteller M. BRCA1 epigenetic inactivation predicts sensitivity to platinum-based chemotherapy in breast and ovarian cancer. Epigenetics. (2012);7:1225–1229. doi: 10.4161/epi.22561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin C, Zhao XY, Li L, et al. NOXA-induced alterations in the Bax/Smac axis enhance sensitivity of ovarian cancer cells to Cisplatin. PLoS One. (2012);7:e36722. doi: 10.1371/journal.pone.0036722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson SW, Laub PB, Beesley JS, Ozols RF, Hamilton TC. Increased platinum-DNA damage tolerance is associated with Cisplatin resistance and cross-resistance to various chemotherapeutic agents in unrelated human ovarian cancer cell lines. Cancer Res. (1997);57:850–856. [PubMed] [Google Scholar]
- 7.Siddik ZH. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene. (2003);22:7265–7279. doi: 10.1038/sj.onc.1206933. [DOI] [PubMed] [Google Scholar]
- 8.Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. (2001);104:487–501. doi: 10.1016/S0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- 9.Desbarats J, Birge RB, Mimouni-Rongy M, Weinstein DE, Palerme JS, Newell MK. Fas engagement induces neurite growth through ERK activation and p35 upregulation. Nat Cell Biol. (2003);5:118–125. doi: 10.1038/ncb916. [DOI] [PubMed] [Google Scholar]
- 10.Golubovskaya VM, Kweh FA, Cance WG. Focal adhesion kinase and cancer. Histol Histopathol. (2009);24:503–510. doi: 10.14670/HH-24.503. [DOI] [PubMed] [Google Scholar]
- 11.Kuhajda FP. Fatty acid synthase and cancer: new application of an old pathway. Cancer Res. (2006);66:5977–5980. doi: 10.1158/0008-5472.CAN-05-4673. [DOI] [PubMed] [Google Scholar]
- 12.Halder J, Lin YG, Merritt WM, et al. Therapeutic efficacy of a novel focal adhesion kinase inhibitor TAE226 in ovarian carcinoma. Cancer Res. (2007);67:10976–10983. doi: 10.1158/0008-5472.CAN-07-2667. [DOI] [PubMed] [Google Scholar]
- 13.Zhou W, Han WF, Landree LE, et al. Fatty acid synthase inhibition activates AMP-activated protein kinase in SKOV3 human ovarian cancer cells. Cancer Res. (2007);67:2964–2971. doi: 10.1158/0008-5472.CAN-06-3439. [DOI] [PubMed] [Google Scholar]
- 14.Wu W, Wang HD, Guo W, et al. Up-regulation of Fas reverses Cisplatin resistance of human small cell lung cancer cells. J Exp Clin Cancer Res. (2010);29:49. doi: 10.1186/1756-9966-29-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu WH, Chang LS. Fas/FasL-dependent and -independent activation of caspase-8 in doxorubicin-treated human breast cancer MCF-7 cells: ADAM10 down-regulation activates Fas/FasL signaling pathway. Int J Biochem Cell Biol. (2011);43:1708–1719. doi: 10.1016/j.biocel.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 16.Cai X, Stoicov C, Li H, et al. Overcoming Fas-mediated apoptosis accelerates Helicobacter-induced gastric cancer in mice. Cancer Res. (2005);65:10912–10920. doi: 10.1158/0008-5472.CAN-05-1802. [DOI] [PubMed] [Google Scholar]
- 17.Bivona TG, Hieronymus H, Parker J, et al. FAS and NF-kappaB signalling modulate dependence of lung cancers on mutant EGFR[J]. Nature. (2011);471:523–526. doi: 10.1038/nature09870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fauvet R, Dufournet C, Poncelet C, Uzan C, Hugol D, Daraï E. Expression of pro-apoptotic (p53, p21, bax, bak and fas) and anti-apoptotic (bcl-2 and bcl-x) proteins in serous versus mucinous borderline ovarian tumours. J Surg Oncol. (2005);92:337–343. doi: 10.1002/jso.20424. [DOI] [PubMed] [Google Scholar]
- 19.Zhou W, Han WF, Landree LE, et al. Fatty acid synthase inhibition activates AMP-activated protein kinase in SKOV3 human ovarian cancer cells. Cancer Res. (2007);67:2964–2971. doi: 10.1158/0008-5472.CAN-06-3439. [DOI] [PubMed] [Google Scholar]
- 20.Yamaguchi H, Wyckoff J, Condeelis J. Cell migration in tumors. Curr Opin Cell Biol. (2005);17:559–564. doi: 10.1016/j.ceb.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 21.Yap TA, Carden CP, Kaye SB. Beyond chemotherapy: targeted therapies in ovarian cancer. Nat Rev Cancer. (2009);9:167–181. doi: 10.1038/nrc2583. [DOI] [PubMed] [Google Scholar]
- 22.Behrens BC, Hamilton TC, Masuda H, et al. Characterization of a cis-diamminedichloroplatinum(II)- resistant human ovarian cancer cell line and its use in evaluation of platinum analogues. Cancer Res. (1987);47:414–418. [PubMed] [Google Scholar]
- 23.Caldecott KW, Tucker JD, Thompson LH. Construction of human XRCC1 minigenes that fully correct the CHO DNA repair mutant EM9. Nucleic Acids Res. (1992);20:4575–4579. doi: 10.1093/nar/20.17.4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li P, Lin Y, Zhang Y, Zhu Z, Huo K. SSX2IP promotes metastasis and chemotherapeutic resistance of hepatocellular carcinoma. J Transl Med. (2013);11:52. doi: 10.1186/1479-5876-11-52. [DOI] [PMC free article] [PubMed] [Google Scholar]