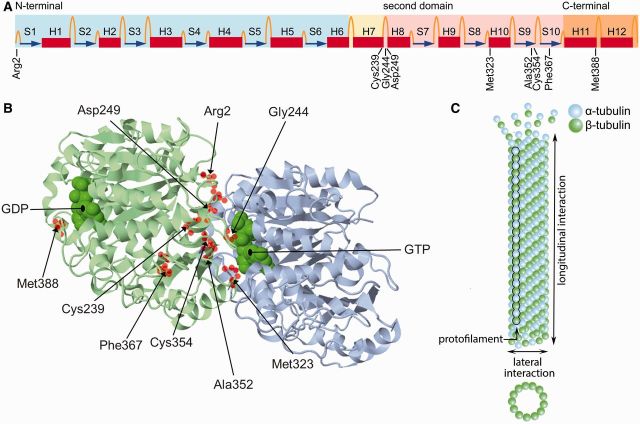
Figure 2.
Structure of tubulin. (A) Linear representation of the secondary structure of β-tubulin (Lowe et al., 2001), showing the positions of the amino acids mutated in the H-ABC patients described here. Coloured boxes indicate the 3D structural domains: the globular N-terminal nucleotide binding domain is blue, the core helix is yellow, the second domain is pink and the C-terminal outer surface domain is orange. Helices are shown as red boxes, β-sheets as blue arrows, and loops as orange threads. Modified from Wade, with permission of Springer (Wade et al., 2009) and from Amos, with permission of Elsevier (Amos and Lowe, 1999). (B) 3D structure of the αβ-tubulin heterodimer (Lowe et al., 2001). The β-tubulin is shown in blue, the α-tubulin in green. Helices, β-sheets and loops are shown as ribbons, arrows and threads, respectively. The mutated residues are highlighted as red spheres. Most mutated residues are located at the intradimer interface. Met388 is located at the interdimer interface. (C) αβ-Tubulin heterodimers bind head-to-tail along their longitudinal direction into protofilaments, which then laterally interact with each other to form microtubules. Modified from Vydra and Havelka (2012).