Abstract
Intranodal lymphangiography (IL) has supplanted pedal lymphangiography (PL) as an easier and more practical approach to opacifying lymphatic vessels for interventional radiologists treating refractory chyle leaks. As more interventional radiologists—many of who are not trained in or have not performed PL—incorporate IL into their practice, it is imperative that they be familiar with the risks of lymphangiography, including pulmonary and systemic embolization of oily contrast material. Herein, the authors report a devastating case of cerebral embolization of ethiodized oil following IL and review the literature regarding systemic embolization following lymphangiography.
Keywords: interventional radiology, stroke, complication, lymphangiogram
Objectives: Upon completion of this article, the reader will be able to identify the potential for complications of lymphangiography, including stroke, and the potential mechanisms for such complications.
Accreditation: This activity has been planned and implemented in accordance with the Essential Areas and Policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint providership of Tufts University School of Medicine (TUSM) and Thieme Medical Publishers, New York. TUSM is accredited by the ACCME to provide continuing medical education for physicians.
Credit: Tufts University School of Medicine designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Intranodal lymphangiography (IL) has supplanted pedal lymphangiography (PL) as an easier and more practical approach to opacifying lymphatic vessels for interventional radiologists treating refractory chyle leaks.1 2 This safer and faster technique has recently renewed interest in lymphangiography,2 especially since PL—a technically challenging and time-intensive procedure that gained popularity in the 1960s and 1970s—had been rendered all but obsolete by advances in noninvasive imaging.3
Today, as more interventional radiologists—many of who are not trained in or have not performed PL—incorporate IL into their practice, it is imperative that they be familiar with the risks of lymphangiography.4 While the intranodal technique eliminates complications associated with a pedal incision, the risks related to the dose of oily contrast material administered, such as pulmonary and systemic embolization, remain. Herein, a devastating case of cerebral embolization of ethiodized oil following IL is reported, followed by a review of the current literature regarding systemic embolization following lymphangiography.
Case Report
A 64-year-old woman with a history of hypertension, diabetes mellitus, latent tuberculosis treated with isoniazid, and a remote history of breast cancer presented to an outside hospital with complaints of chronic diarrhea and a palpable abdominal mass. Computerized tomography (CT) scan of the abdomen demonstrated a large, calcified mesenteric mass surrounded by numerous mildly enlarged lymph nodes (not shown). The patient underwent an exploratory laparoscopy with peritoneal washings and intraoperative omental and peritoneal biopsies. The surgical pathology demonstrated sclerosing mesenteritis with no evidence of malignancy or infection. Following surgery, corticosteroid therapy was initiated. However, she developed worsening abdominal distention requiring multiple large-volume paracenteses for rapidly reaccumulating chylous ascites. She was referred to the authors' institution for further management.
The patient was initially evaluated by Gastroenterology and Infectious Disease physicians, and was treated medically for failure to thrive and recurrent chylous ascites. Despite conservative medical management, including diuretic therapy and empiric treatment for presumed peritoneal tuberculosis, the patient's symptoms did not improve.5 6 During a subsequent inpatient hospitalization, interventional radiology was consulted to perform a lymphangiogram to identify and possibly treat the source of refractory chylous ascites.
IL was utilized to perform the lymphangiogram. Briefly, after the administration of local anesthesia, 25-gauge needles were placed within bilateral inguinal lymph nodes using ultrasound guidance. Approximately 25+ mL of ethiodized oil (Lipiodol Ultra-Fluide; Guerbet, France) was directly injected into the lymph nodes, resulting in opacification of the lymph nodes and efferent lymphatics. If extravasation was observed during injection, the needle position was adjusted or a different lymph node was accessed. Lymphangiography opacified the pelvic and abdominal lymphatic vessels and the thoracic duct, but did not demonstrate extravasation or a source of the patients chylous ascites (Fig. 1). Following the procedure, the patient left the interventional radiology procedure suite in stable condition.
Fig. 1.
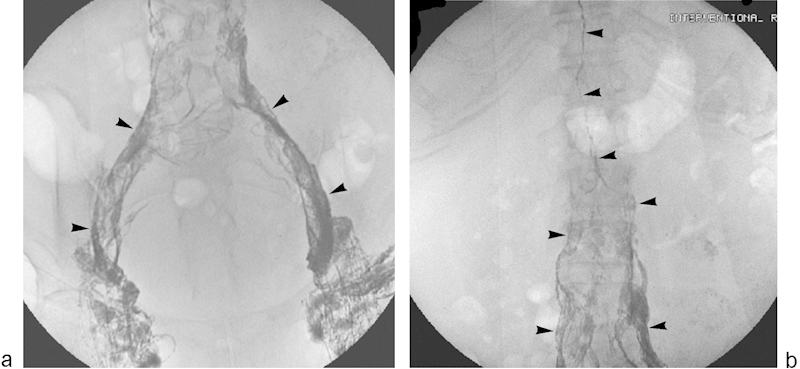
Representative images from direct pelvic intranodal lymphangiography. Fluoroscopic spot images of the pelvis (a) and abdomen (b) following direct intranodal ethiodized oil injection demonstrate filling of pelvic and abdominal lymphatics (arrowheads).
That night, however, the patient had an unwitnessed fall. A CT scan of the head was obtained and revealed small high attenuation foci scattered within the bilateral basal ganglia and cortical gray matter (Fig. 2), consistent with embolization of ethiodized oil to the brain. These findings prompted a chest X-ray (Fig. 3) that demonstrated ethiodized oil deposition within the pulmonary vasculature. An echocardiogram showed no evidence for right-to-left shunting in the heart. Over the next few days, the patient became confused with progressively declining mental status. On postprocedure day 9, the patient became unresponsive, and electroencephalography revealed that the patient was in generalized status epilepticus. The patient required numerous antiepileptic medications for seizure control. On postprocedure day 18, the patient's mental status had improved slightly and she was alert and responsive but nonverbal. However, her mental status deteriorated and she again became unresponsive. Her hospital course was further complicated by pneumonia requiring several admissions to the intensive care unit. Despite aggressive medical management, the patient remained in a persistent vegetative state for weeks. With little chance of a meaningful recovery, the patient's family decided to withdraw all supportive care, and the patient expired 69 days after the lymphangiogram.
Fig. 2.
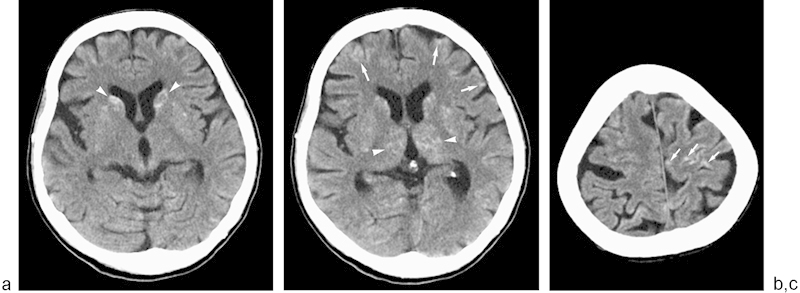
Cerebral embolization of ethiodized oil. (a–c) Noncontrast head CT scans reveal high attenuation foci of ethiodized oil scattered within the bilateral basal ganglia (arrowheads) and cortical gray matter (arrows).
Fig. 3.
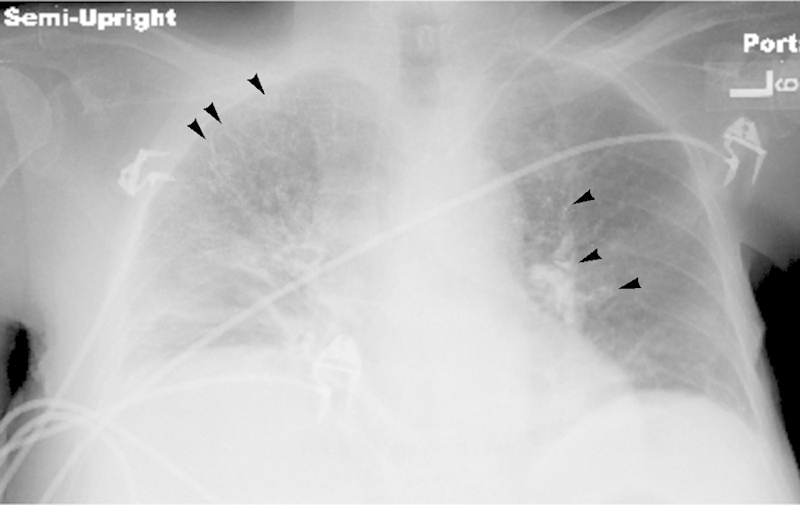
Ethiodized oil deposition within pulmonary vasculature. Frontal chest radiograph reveals abundant high attenuation material (arrowheads) distributed throughout the distal pulmonary arteries bilaterally, consistent with pulmonary arterial embolization of ethiodized oil.
Autopsy confirmed embolized lipid material present in and around occasional scattered blood vessels in the brain stem, basal ganglia, and cerebral cortex in association with focal ischemic changes. Additionally, scattered foci of embolized lipid material were present in lung.
Discussion
In 1998, Constantin Cope first described percutaneous transabdominal catheterization of lymphatic vessels to treat chyle leaks.7 Since then, percutaneous image-guided treatment of chyle leaks has been established as a safe and effective procedure.8 However, until recently, few interventional radiologists were performing these procedures because the most common method of opacifying the lymphatic vessels—PL—was a technically challenging, time-consuming procedure that had been rendered all but obsolete. Indeed, over the past several years few interventional radiologists have been trained in PL. Following two recent reports demonstrating the feasibility and safety of IL,1 2 interventional radiologists now have an easier and more practical approach to lymphangiography that will likely lead to an increase in its use.
Today's interventional radiologists, who likely have little experience with lymphangiography, should be familiar with the risks of this procedure, including pulmonary and systemic embolization of the ethiodized oil used for lymphatic vessel opacification. Numerous case reports dating back to more than 40 years describe these risks.9 10 11 Strict contraindications to lymphangiography include pulmonary insufficiency and a known right-to-left cardiac shunt. The former predisposes the patient to respiratory complications as the lipid droplets obstruct the capillary beds of the pulmonary arteries; the latter presents a risk of systemic arterial embolization of the lipid material. Patients undergoing lymphangiography will usually develop ethiodized oil pulmonary emboli as the pulmonary capillaries filter the lipid molecules that are introduced into the venous system when the thoracic duct empties into the subclavian vein. At low doses these are usually not clinically significant; however, at higher doses these emboli can lead to respiratory complications.11
In the case presented here, cerebral embolization of ethiodized oil was noted following IL that likely contributed to devastating neurologic sequelae including generalized status epilepticus and a persistent vegetative state. These clinical sequelae occurred despite premortem and postmortem studies documenting no identifiable right-to-left shunt. In the absence of cardiac shunts, the mechanism of arterial lipid emboli following lymphangiography is not well understood.
Almost 50 years ago, Nelson et al11 reported lipid embolization to the brain after lymphangiography, and numerous additional reports have described neurological radiation changes following lymphangiography.9 10 12 13 14 15 There are several proposed mechanisms for this phenomenon. The first involves changes in the pulmonary vasculature and subsequent disruption of the filtering ability of the pulmonary arteries and capillaries, allowing lipid emboli into the systemic circulation.9 16 This was confirmed in animal models where irradiated rabbits developed systemic arterial emboli following pulmonary vessel lipiodol embolization.9 This mechanism was proposed after a patient with radiation therapy to the chest developed systemic emboli after lymphangiography.9 Although the patient described here had a remote history of breast cancer, she had no documented history of radiation therapy.
Another proposed mechanism involves the rapid accumulation of lipid emboli in the pulmonary vasculature, “overloading” the vessels and resulting in systemic emboli. Lastly, pulmonary arteriovenous (AV) shunts can predispose the patient to systemic arterial embolization. Most pulmonary AV shunts are hereditary and noted in patients with hereditary hemorrhagic telangiectasia; however, enlarged acquired shunts may also be noted in patients with hepatopulmonary syndrome, malignancy, and schistosomiasis.17 18
In addition to its use in lymphangiography, ethiodized oil is also used as a contrast agent in hysterosalpingography and is routinely used as an embolic agent in transarterial chemoembolization (TACE), most commonly for primary liver cancer.19 There are multiple case reports of systemic embolization of ethiodized oil after these procedures.20 21 22 In these cases, the mechanisms of embolization described include lymphovenous or AV shunts. The risk of symptomatic ethiodized oil pulmonary emboli following TACE is directly related to the volume of ethiodized oil used. Chung et al reported symptomatic pulmonary oil embolization following TACE in patients in whom more than 20 mL of ethiodized oil was administered.23
The ideal dose of ethiodized oil for lymphangiography can be derived from the PL literature. In 1964, Gough24 recommended 0.25 mL/kg body weight as a maximum dose of ethiodized oil. Based on this recommendation, the maximum dose of ethiodized oil for the patient presented here should have been 15.4 mL (based on her weight of 61.5 kg). Instead, she received approximately 20 to 24 mL of ethiodized oil, which likely increased her risk for clinically significant emboli. In 1982, Fyfe recommended that bipedal lymphangiography be performed with 5 to 7 mL of ethiodized oil per extremity.25 Symptomatic pulmonary embolism may occur with larger volumes of ethiodized oil.25 Animal studies of lymphangiography show that the lungs contain 50% of the total volume of injected ethiodized oil, the lymphatics retain 25%, and the rest is distributed in smaller concentrations throughout the body.26 The presence of even a small amount of ethiodized oil in other organs supports the presumption that larger doses of ethiodized oil predispose an individual to a higher risk of clinically significant emboli.
Summary
IL has supplanted PL as an easier and more practical approach to opacifying lymphatic vessels for interventional radiologists treating refractory chyle leaks. As more interventional radiologists—many of whom were neither trained in nor perform PL—incorporate IL into their practice, it is imperative that they be familiar with the risks of lymphangiography, including pulmonary and systemic embolization of ethiodized oil. Although rare, these complications can result in devastating clinical outcomes, highlighted by the current case of cerebral embolization of ethiodized oil following IL. Prior to performing IL, interventional radiologists should obtain a thorough history, including a history of right-to-left vascular shunt, radiation to the chest, or any underlying comorbidity that may reduce the efficacy of the pulmonary arteries to filter ethiodized oil emboli or predispose the patient to pulmonary AV shunts. Additionally, practitioners should rely on intermittent fluoroscopic observation of the lymphatic contrast medium to help prevent excessive administration of ethiodized oil, as well as limiting administration to 15 to 20 mL since higher doses may predispose the patient to clinically significant pulmonary and systemic emboli.
References
- 1.Rajebi M R, Chaudry G, Padua H M. et al. Intranodal lymphangiography: feasibility and preliminary experience in children. J Vasc Interv Radiol. 2011;22(9):1300–1305. doi: 10.1016/j.jvir.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 2.Nadolski G J, Itkin M. Feasibility of ultrasound-guided intranodal lymphangiogram for thoracic duct embolization. J Vasc Interv Radiol. 2012;23(5):613–616. doi: 10.1016/j.jvir.2012.01.078. [DOI] [PubMed] [Google Scholar]
- 3.Strijk S P, Boetes C, Rosenbusch G, Ruijs J H. Lymphography and abdominal computed tomography in staging Hodgkin's disease. Rofo. 1987;146(3):312–318. doi: 10.1055/s-2008-1048489. [DOI] [PubMed] [Google Scholar]
- 4.Cope C, Kaiser L R. Management of unremitting chylothorax by percutaneous embolization and blockage of retroperitoneal lymphatic vessels in 42 patients. J Vasc Interv Radiol. 2002;13(11):1139–1148. doi: 10.1016/s1051-0443(07)61956-3. [DOI] [PubMed] [Google Scholar]
- 5.Emınler A T, Ayyildiz T, Irak K, Dolar E. Tuberculous peritonitis case at advanced age presenting with chylous ascites. Turk J Gastroenterol. 2012;23(4):423–425. doi: 10.4318/tjg.2012.0382. [DOI] [PubMed] [Google Scholar]
- 6.Arora M, Dubin E. A clinical case study: sclerosing mesenteritis presenting as chylous ascites. Medscape J Med. 2008;10(2):30. [PMC free article] [PubMed] [Google Scholar]
- 7.Cope C. Diagnosis and treatment of postoperative chyle leakage via percutaneous transabdominal catheterization of the cisterna chyli: a preliminary study. J Vasc Interv Radiol. 1998;9(5):727–734. doi: 10.1016/s1051-0443(98)70382-3. [DOI] [PubMed] [Google Scholar]
- 8.Itkin M Kucharczuk J C Kwak A Trerotola S O Kaiser L R Nonoperative thoracic duct embolization for traumatic thoracic duct leak: experience in 109 patients J Thorac Cardiovasc Surg 20101393584–589., discussion 589–590 [DOI] [PubMed] [Google Scholar]
- 9.Davidson J W. Lipid embolism to the brain following lymphography. Case report and experimental study. Am J Roentgenol Radium Ther Nucl Med. 1969;105(4):763–771. doi: 10.2214/ajr.105.4.763. [DOI] [PubMed] [Google Scholar]
- 10.Moskowitz G, Chen P, Adams D F. Lipid embolization to the kidney and brain after lymphangiography. Radiology. 1972;102(2):327–328. doi: 10.1148/102.2.327. [DOI] [PubMed] [Google Scholar]
- 11.Nelson B, Rush E A, Takasugi M, Wittenberg J. Lipid embolism to the brain after lymphography. N Engl J Med. 1965;273(21):1132–1134. doi: 10.1056/NEJM196511182732105. [DOI] [PubMed] [Google Scholar]
- 12.Winterer J T, Blum U, Boos S, Konstantinides S, Langer M. Cerebral and renal embolization after lymphography in a patient with non-Hodgkin lymphoma: case report. Radiology. 1999;210(2):381–383. doi: 10.1148/radiology.210.2.r99fe09381. [DOI] [PubMed] [Google Scholar]
- 13.Kusumoto S, Imamura A, Watanabe K. Case report: the incidental lipid embolization to the brain and kidney after lymphography in a patient with malignant lymphoma: CT findings. Clin Radiol. 1991;44(4):279–280. doi: 10.1016/s0009-9260(05)80199-0. [DOI] [PubMed] [Google Scholar]
- 14.de Munck B, Bourgeois P, Vanderperre H, Vermeulen J, Clarysse H J, Demeester J. [Fatty cerebral embolism after lymphography] Rev Neurol (Paris) 1988;144(2):127–130. [PubMed] [Google Scholar]
- 15.Sane D C, Massey E W, Moore J. Lipid cerebral embolization following lymphogram. Clin Neuropharmacol. 1985;8(2):184–188. doi: 10.1097/00002826-198506000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Cammermeyer J, Swank R L. Acute cerebral changes in experimental canine fat embolism. Exp Neurol. 1959;1:214–232. doi: 10.1016/0014-4886(59)90002-0. [DOI] [PubMed] [Google Scholar]
- 17.Cartin-Ceba R, Swanson K L, Krowka M J. Pulmonary arteriovenous malformations. Chest. 2013;144(3):1033–1044. doi: 10.1378/chest.12-0924. [DOI] [PubMed] [Google Scholar]
- 18.Kopetz S, Jimenez C, Tu S-M, Sharma P. Pulmonary arteriovenous fistula in a patient with renal cell carcinoma. Eur Respir J. 2007;29(4):813–815. doi: 10.1183/09031936.00077106. [DOI] [PubMed] [Google Scholar]
- 19.Bruix J Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update Hepatology 20115331020–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Charawanamuttu A M, Hughes-Nurse J, Hamlett J D. Retinal embolism after hysterosalpingography. Br J Ophthalmol. 1973;57(3):166–169. doi: 10.1136/bjo.57.3.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi C-S, Kim K H, Seo G S. et al. Cerebral and pulmonary embolisms after transcatheter arterial chemoembolization for hepatocellular carcinoma. World J Gastroenterol. 2008;14(30):4834–4837. doi: 10.3748/wjg.14.4834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uzun O, Findik S, Danaci M, Katar D, Erkan L. Pulmonary and cerebral oil embolism after hysterosalpingography with oil soluble contrast medium. Respirology. 2004;9(1):134–136. doi: 10.1111/j.1440-1843.2003.00524.x. [DOI] [PubMed] [Google Scholar]
- 23.Chung J W, Park J H, Im J G, Han J K, Han M C. Pulmonary oil embolism after transcatheter oily chemoembolization of hepatocellular carcinoma. Radiology. 1993;187(3):689–693. doi: 10.1148/radiology.187.3.8388567. [DOI] [PubMed] [Google Scholar]
- 24.Gough M H. Lymphangiography in children. Arch Dis Child. 1964;39:177–181. doi: 10.1136/adc.39.204.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fyfe N C, Rutt D L, Edwards J M, Kinmonth J B. Intralymphatic steroid therapy for lymphoedema: preliminary studies. Lymphology. 1982;15(1):13–28. [PubMed] [Google Scholar]
- 26.Koehler P R, Meyers W A, Skelley J F, Schaffer B. Body distribution of ethiodol following lymphangiography. Radiology. 1964;82:866–871. doi: 10.1148/82.5.866. [DOI] [PubMed] [Google Scholar]


