Abstract
This study investigated the consequence of repeated stress on actin cytoskeleton remodeling in the nucleus accumbens (NAc) and prefrontal cortex (Pfc), and the involvement of this remodeling in the expression of stress-induced motor cross-sensitization with cocaine. Wistar rats were restrained daily (2 hours) for 7 days and, three weeks later, their NAc and Pfc were dissected 45 min after acute saline or cocaine (30 mg/kg i.p.). F-actin, actin-binding proteins (ABP) and GluR1 were quantified by western blotting, and dendritic spines and PSD size measured by electron microscopy. In the NAc from the stress plus cocaine group we observed a decrease in the phosphorylation of two ABPs, cofilin and cortactin, and an increase in the PSD size and the surface expression of GluR1, consistent with a more highly branched actin cytoskeleton. The Pfc also showed evidence of increased actin polymerization after stress as an increase was observed in Arp2, and in the number of spines. Inhibiting actin cycling and polymerization with latrunculin A into the NAc, but not the Pfc, inhibited the expression of cross-sensitization to cocaine (15 mg/kg i.p.) and restored the expression of GluR1 to control levels. This study shows that a history of repeated stress alters the ability of a subsequent cocaine injection to modulate dendritic spine morphology, actin dynamics and GluR1 expression in the NAc. Furthermore, by regulating GluR1 expression in the NAc, elevated actin cycling contributes to the expression of cross-sensitization between stress and cocaine, while stress-induced changes in the Pfc were not associated with cross-sensitization.
Keywords: F-actin, actin binding proteins, dendritic spines, AMPA receptors, latrunculin A
Introduction
It is well known that there is a high rate of co-occurrence of substance abuse disorder and stressful life experiences (Brady & Sinha, 2005). In animal models, exposure to stress results in long-term changes in stress and drug responses (Lu et al., 2003; Marinelli & Piazza 2002). Behavioral sensitization is an example of experience-dependent plasticity, induced by drug or stress, which has been suggested to involve plasticity at glutamatergic synapses (Kauer & Malenka, 2007; Kalivas, 2009), and there is evidence for a common mechanism triggered by stress and drugs at excitatory synapses on midbrain dopamine neurons (Saal et al., 2003; Ungless et al., 2003).
Morphological changes in dendritic spines have been associated with behavioral adaptations to motivationally relevant stimuli (Bhatt et al., 2009; Kalivas et al., 2009). Like repeated drug administration, repeated exposure to stressors induces enduring adaptations in dendritic branching, shape and the number of spines (Robinson & Kolb, 1997; 1999; 2004; Brown et al., 2005; Shen et al., 2009), as well as in postsynaptic density (PSD) proteins (Swanson et al., 2001; Yao et al., 2004; Szumlinski et al., 2008). Importantly, repeated stress pretreatment increases the locomotor response to an acute cocaine injection, a phenomenon referred to as cross-sensitization (Prasad et al., 1998). However, the role of stress-induced changes in the neuronal architecture in the cross-sensitization between stress and cocaine is unknown.
Dendritic spine morphology and protein insertion into the PSD are regulated by actin cycling (Krause et al., 2003; McGee & Bredt, 2003; Matus, 2005), which is controlled by various actin-binding proteins (ABPs) that maintain equilibrium between monomeric [G (globular)] and filamentous [F (filamentous)] actin (dos Remedios et al., 2003). Both the polymerization and depolymerization of F-actin are modulated, in part, by phosphorylation of the ABPs, thereby linking actin dynamics to excitatory neurotransmission and associated kinase signaling cascades (Carlisle & Kennedy, 2005; Cingolani & Goda, 2008). Furthermore, (Toda et al., 2006) showed that repeated cocaine enhances actin cycling in the nucleus accumbens (NAc). Additionally, (Boudreau & Wolf, 2005) found an upregulation of AMPA-type glutamate receptor (AMPAR) surface expression in the NAc of behaviorally sensitized rats after withdrawal from repeated cocaine injections.
This study investigated the consequence of repeated stress on actin cytoskeleton remodeling in the NAc and prefrontal cortex (Pfc) and the involvement of this remodeling in the expression of cross-sensitization to cocaine-induced locomotor activity after prior repeated stress. Specifically, we aimed to evaluate changes in the level and/or phosphorylation state of F-actin and the ABPs regulating actin dynamics, the surface expression of GluR1, and the number of dendritic spines and the size of PSD within the NAc and the Pfc. In addition, inhibition of actin dynamics by latrunculin A in the NAc or Pfc was used to determine whether actin rearrangement influences the expression of behavioral cross-sensitization and the increase in GluR1 in NAc seen after a cocaine injection in rats pretreated with repeated stress. Finally, we extended these studies to assess the capacity of intra-accumbens AMPA antagonist 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) to alter the sensitized motor stimulant response to cocaine following repeated stress.
Methods and Materials
Animals
Male Wistar rats (250–350 g) were immobilized daily for 2 h in a Plexiglass restraining device, for seven consecutive sessions between 10:00 and 14:00 h (Fig. 1A). The Plexiglass cylinders were designed to allow the rats’ tails to emerge from the rear. The animals appeared healthy as evidenced by their coat texture and minimal, reversible changes in body weight (Cancela et al., 1996). Control animals remained undisturbed in their home cages for the full 28-day period except for weekly weighing and regular husbandry. Three weeks after the last restraint stress, the biochemical, behavioral and electron microscopic (EM) studies were performed. All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals as approved by the Animal Care and Use Committee of the Facultad de Ciencias Químicas, Universidad Nacional de Córdoba.
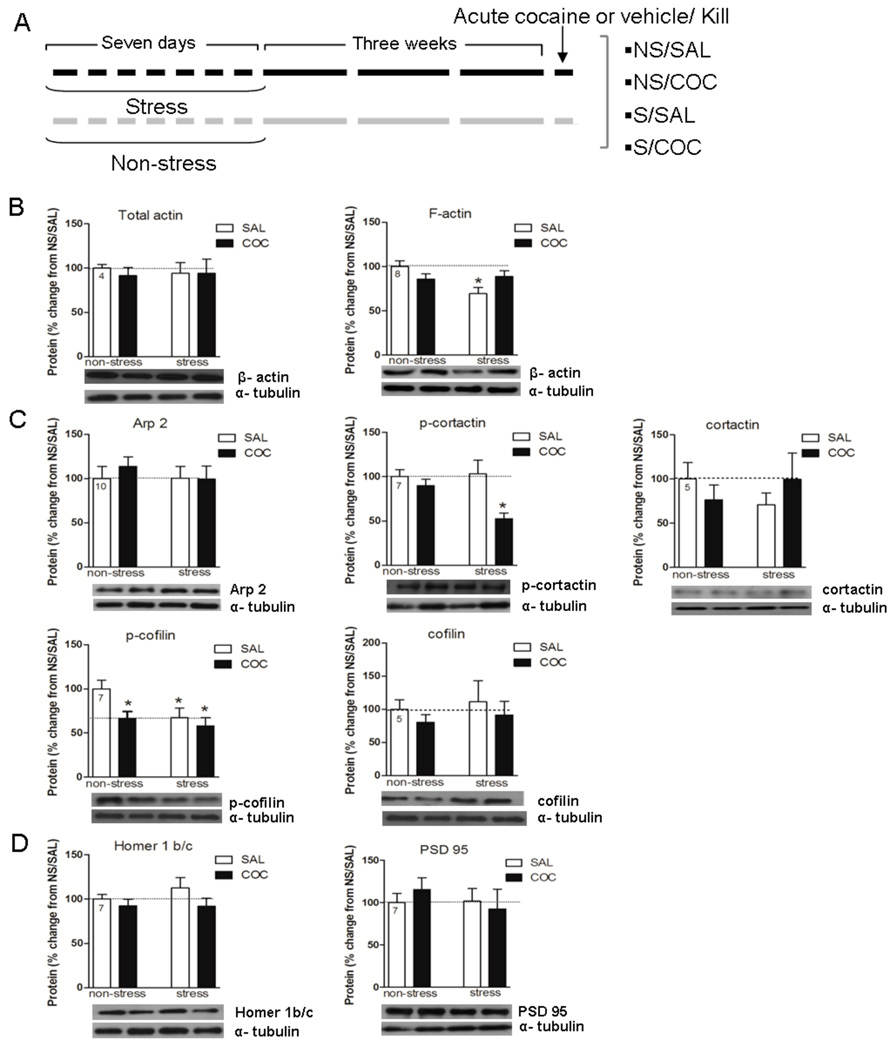
Figure 1.
A, Stress treatment regimens: NS/SAL, non-stress/acute saline; NS/COC, non-stress/acute cocaine; S/SAL, repeated stress/acute saline; S/COC repeated stress/acute cocaine. B, C, and D, Effects of repeated stress and cocaine on levels of F-actin, ABPs (actin-bound proteins) and PSD proteins in the NAc. Data is expressed as mean ± SEM of the number of determinations depicted in each graphic. The results were analyzed by a two-way ANOVA (stress × drug) and a Tukey´s test as a post hoc comparison when a significant interaction was present. B, F-actin, stress × drug F(1,28)=6.54, p=0.016. * p<0.05 compared to NS/SAL. C, p-cofilin, stress F(1,24)=4.77, p=0.039; drug F(1,24)=5.12, p=0.032. * p<0.05 compared to NS/SAL. p-cortactin, stress × drug F(1,22)=4.63, p=0.042. * p<0.05 compared to all the remaining groups.
Biochemical Studies
Subcellular Fractionation and Immunoblotting
Animals were decapitated 45 minutes after an acute injection of saline or cocaine (30 mg/kg i.p.), three weeks after the last restraint stress (as described above). Brains were rapidly removed and 2 mm coronal sections containing the Pfc, or striatum and NAc were obtained using a brain matrix on an ice-cold platform. The dissected slice of NAc tissue contained both the core and shell subregions, although there was relatively more core tissue. The dissected slice of the prefrontal cortex included the dorsal region. After finishing the dissections, bilateral NAc, Pfc and striatum tissue blocks from two animals were pooled to obtain sufficient material for western blotting. Subcellular fractionation was performed on dissected brain tissue as described in detail previously (Toda et al., 2006). The NAc, striatum and Pfc tissues were homogenized in cold buffer containing 0.32 M sucrose, 10 mM HEPES, 1 mM EDTA, pH 7.4 and assorted protease and phosphatase inhibitors. Homogenates were centrifuged three times at 1,000 × g for 10 min to separate the nuclear fraction. The resulting supernatants were concentrated twice at 10,000 × g for 15 min to obtain a crude synaptosomal fraction. This fraction was resuspended in ice-cold water for hypoosmotic shock to release synaptic vesicles from the membrane, then rapidly adjusted to pH 7.4 with 4 mM HEPES buffer and incubated for 45 min with gentle rotation. After a 20 min centrifugation at 25,000 × g, the supernatant was discarded and the resulting pellet (the synaptosomal membrane fraction) was resuspended in buffer A (1% Triton-X, 20 mM HEPES pH 7.2, 100 mM NaCl) and incubated at 4°C for 1 h with gentle rotation. After a 20 min centrifugation (10,000 × g, at 4°C), the pellet was resuspended in buffer B (15 mM HEPES NaOH, 0.15 mM NaCl, 1% SDS, 10 mM EDTA, and 1 mM DTT, pH 7.5), and the supernatant collected as the non-PSD membrane fraction (which contains the G-actin fraction). The supernatant resulting from 1 h of the pellet incubation at 4°C and an additional 20 min centrifugation (10,000 × g, at 4°C) was recovered as the PSD fraction (F-actin enriched fraction, approx. 0.5 µg/µl was obtained from two animal tissues). Loaded samples containing 25 µg for the F-actin fraction and 40 µg for the G-actin fraction of protein were detected by western-blot using tubulin as a loading control. A total of 80 animals were used in this experiment. The immunoreactivity of actin (n=6–9/group), PSD-95 (n=7–8/group), Arp 2 (n=10/group), Homer1b/c (n=6–7/group), p-cortactin (n=7–8/group) and cortactin (n=5–7/group) were detected from the F-actin fraction, and the immunoreactivity of p-cofilin (n=7/group) and cofilin (n=6–7/group) were detected from the G-actin fraction. A recent report illustrates the relative capacity of this subfractionation procedure to separate PSD from non-PSD proteins (Knackstedt et al., 2010).
Surface Biotinylation
AMPAR expression in the NAc
Three weeks after discontinuing repeated stress, rats were injected i.p. with saline, or cocaine 30 mg/kg and sacrificed 45 min later and the expression of GluR1 was examined (n=5/group). In another experiment, three weeks after discontinuing repeated stress, the animals were injected intra-accumbens with Latrunculin A (0.5 µg) or vehicle and 5 min later administered cocaine (30 mg/kg i.p.) and sacrificed 45 min later to examine the expression of GluR1 (n=4–5/group). The NAc (including both shell and core subcompartments, although there was relatively more core tissue) was dissected. Bilateral NAc tissue blocks from two animals were merged to obtain enough material for surface expression. A total of 76 animals were used in this experiment The dissected area was transferred to ice-cold sulfo-NHS-LC-biotin (Pierce, Rockford, IL) in PBS (0.3 mg/ml) and incubated for 1 h, then rinsed in cold Tris-glycine to quench free biotin (5 min) followed by washes with ice-cold TBS (3×5 min). Microdissected NAc was homogenized in 300 µl of RIPA buffer. Homogenates were centrifuged at 13,000 × g for 30 min to pellet the insoluble fraction. For the total fraction of GluR1 (surface plus internal), 50 µl of the supernatant was mixed and heated with 4× SDS sample buffer. Biotinylated surface proteins in the remaining supernatant (200 µl) were immunoprecipitated with 50 µl of 50% avidin-agarose beads (ImmunoPure Immobilized Avidin; Pierce, Rockford, IL) for 2 h at 4°C. The beads were pelleted, and 150 µl of the supernatant (internal fraction) was mixed and heated with 4× SDS sample buffer. The beads were then rinsed three times with ice-cold TBS and heated in 50 µl of 2× SDS sample buffer (surface fraction). The surface fraction and surface plus internal were subjected to quantitative immunoblotting for AMPAR using anti-GluR1 (AB 1504), Millipore, Billerica, MA). In the GluR1 plasma membrane expression study, plasma membrane-associated AMPAR was defined as the surface fraction of SDS sample buffer eluent collected from avidin beads, normalized to the total fraction of GluR1 (surface plus internal, which represents total GluR1 present prior to avidin bead addition) in each sample.
Behavioral Study
Surgery and Microinjection
Two weeks after the last restraint stress, all animals were stereotaxically implanted with guide cannulas under ketamine/xylazine anesthesia (55 mg/kg and 11 mg/kg, respectively) in the NAc at +0.6 mm anterior, ±1.8 mm lateral (at an angle of 6° from vertical), and -4.0 mm ventral from bregma or in the Pfc at +3.2 mm anterior, ±0.3 mm lateral, and -3.4 mm ventral from bregma (Paxinos & Watson, 2009). For latrunculin A microinjection: One week after surgery, bilateral infusion cannulas were inserted to microinfuse latrunculin A (Invitrogen, Carlsbad, CA) dissolved in DMSO (1%) in a volume of 1 µl for 2 min (n=6–7/group). A total of 55 animals were used for latrunculin experiments in NAc and Pfc. For CNQX microinjection: One week after surgery, bilateral infusion cannulas were inserted to microinfuse CNQX (Sigma, St. Louis, MO) dissolved in DMSO (10%) in a volume of 0.5 µl for 1 min. An additional 2 min were allowed for diffusion before the infusion cannulas were removed (n=9–10/group). A total of 34 animals were used for CNQX experiment. For behavioral studies, 0.5 µg of latrunculin A or vehicle (DMSO 1%), or 1 nmol of CNQX or vehicle (DMSO 10%) were microinjected 5 min before a systemic injection of either cocaine or saline, in the same conditions described above. The dose of latrunculin employed (0.5 µg/µl) was selected in accordance with that used by Toda et al., (Toda et al., 2006; Toda et al., 2010), who selected this dose based in part in the “in vitro” literature (Fischer et al., 2004) but also in preliminary “in vivo” studies showing effects of different microinjected latrunculin doses on F-actin in cocaine/saline animals. Furthermore, (Toda et al., 2010) showed that latrunculin A (0.5 µg/µl) injected in NAc abolished the ability of acute cocaine to increase the spine head diameter, and to simultaneously inhibit the expression of the sensitized behavioral response to the drug. The 1 nmol CNQX dose was selected based upon studies of (Pierce et al., 1996) who showed that a pretreatment with 0.1 nmol and 1 nmol CNQX, blocks locomotor sensitization to cocaine , whereas a pretreatment with 0.01 nmol was ineffective. Furthermore, “in vivo” preliminary studies from our lab show that 21 days following a single restraint stress both 0.1 and 1.0 nmol CNQX significantly inhibited the sensitized motor response to cocaine without significantly reducing the acute response to this drug in control animals (Garcia-Keller et al., submitted).
Cocaine-Induced Locomotion
Locomotor activity was monitored in photocell apparatus (actographs) and quantified as total photocell counts (interruption of a beam that resulted in a photocell count). For these sessions, animals were allowed to habituate to the activity chambers for 60 min before the drug (latrunculin A or CNQX) or vehicle (DMSO) microinjections, which were followed by cocaine (15 mg/kg i.p.) or saline, and behavior recorded for 120 min over 10 min interval-bins. It is noteworthy that 15 mg/kg cocaine rather than 30 mg/kg was used, to avoid the manifestation of the typical stereotypic response to larger doses of cocaine.
Electron Microscopy
Three weeks after the last restraint stress session, all animals were perfused 45 min after an acute injection of saline or cocaine (30 mg/kg i.p.). A total of 18 animals (n=3–5/group) were fixed by perfusion with 4% wt/v paraformaldehyde plus 2.5% v/v glutaraldehyde in 0.1 M phosphate buffer, pH 7.4. Subsequently, 1 mm-thick sections of Pfc (layers 2–4) and NAc core were postfixed with 1.5% wt/v osmium tetroxide in 0.1 mM phosphate buffer pH 7.4 for 2 h at 4°C. The sections were contrasted with 2% wt/v uranyl acetate, dehydrated and embedded in Epon 812 resin. Ultrathin sections were obtained with an ultramicrotome Porter Bloom MT 1 and collected in 300 mesh copper grids. These sections were contrasted with uranyl acetate and stained with Reynolds solution, and then photographed in a Zeiss 10C Electron Microscope using 35-mm Kodak Technical Pan Professional 2415 films. Forty neuropil fields from each area per animal were photographed at 12,500 × primary magnification with an Electron Microscope Zeiss 10C. The films were scanned and digital images were used for counting the dendritic spines involved in axospinous synapses. Axospinous synapses were identified by the presence of an axon terminal with synaptic vesicles in contact with small postsynaptic profiles, which show postsynaptic density and spinous apparatus. EM photographs were digitally processed to measure the postsynaptic density thickness and length in an Image Pro image analyzer. The postsynaptic density thickness was evaluated as the length of a perpendicular line traced from the postsynaptic membrane to the most convex part of the synaptic complex in 50,000 × primary magnification EM photographs. The postsynaptic density length was evaluated as the longitude of a line traced parallel to the postsynaptic membrane in 50,000 × primary magnification EM photographs. The experimenter was blind to the condition.
Drug and Antibodies
Cocaine was purchased from Verardo Laboratories (Buenos Aires, Argentina). The following antibodies were used: a goat polyclonal anti-actin (1:500; Santa Cruz Biotechnology, Santa Cruz, CA), a rabbit polyclonal antibody against phosphorylated cofilin (1:1000; kindly provided by Dr James Bamburg, Colorado State University, Fort Collins, CO), a mouse monoclonal anti-α-tubulin (1:2000, Sigma-Aldrich, St. Louis, MO), a rabbit polyclonal anti-Arp2 (1:100; Santa Cruz Biotechnology, Santa Cruz, CA), a mouse polyclonal anti-Homer 1b/c (1:100; Santa Cruz Biotechnology, Santa Cruz, CA), a goat polyclonal anti-PSD 95 (1:250; Santa Cruz Biotechnology, Santa Cruz, CA), a rabbit polyclonal against phosphorylated cortactin (1:200; Santa Cruz Biotechnology, Santa Cruz, CA), a rabbit polyclonal anti-cortactin (1:250; Cell Signaling, Beverly, MA), a rabbit polyclonal anti-cofilin (1:250; Cell Signaling, Beverly, MA) and a rabbit monoclonal anti-GluR1 (kindly provided by Dr Peter Kalivas 1:400; Millipore). Secondary antibodies were: peroxidase conjugated anti-goat (1:2000; Jackson Laboratories, Baltimore Pike, PA), peroxidase conjugated anti-mouse (1:2000; Jackson Laboratories, Baltimore Pike, PA), and peroxidase conjugated anti-rabbit (1:2000; Jackson Laboratories, Baltimore Pike, PA).
Statistical Analyses
A two-way ANOVA with the following factors: stress (non-stress or stress) and drug (cocaine or saline) (Fig. 1, 2, 4A, 6, 8); or stress (non-stress or stress) and treatment (latrunculin A or vehicle) (Fig.4B) was used to evaluate biochemical and microscopy data. Behavioral data were statistically evaluated using a two-way ANOVA with the following factors: stress (non-stress or stress) and treatment (latrunculin A/ vehicle or CNQX/vehicle) with repeated measures over time (Fig. 3, 7 and 5, repectively). All ANOVA were followed by a Tukey´s test for post hoc comparisons. The level of significance for all procedures was p<0.05.
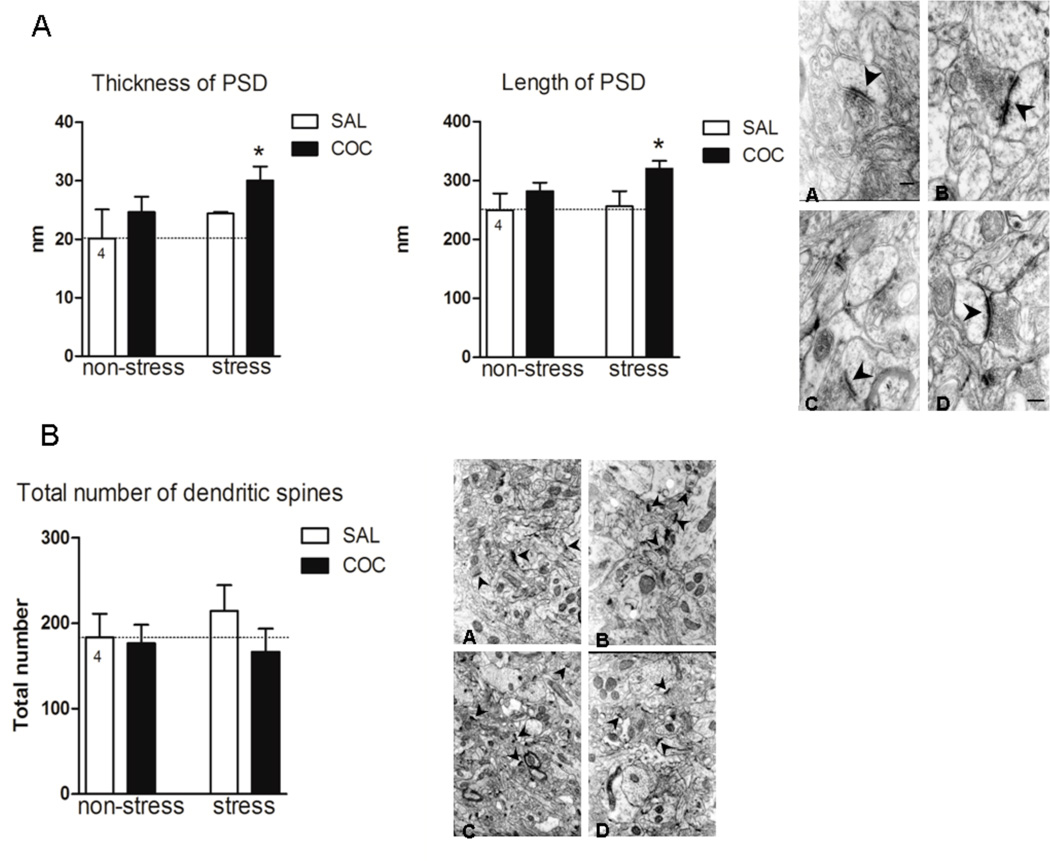
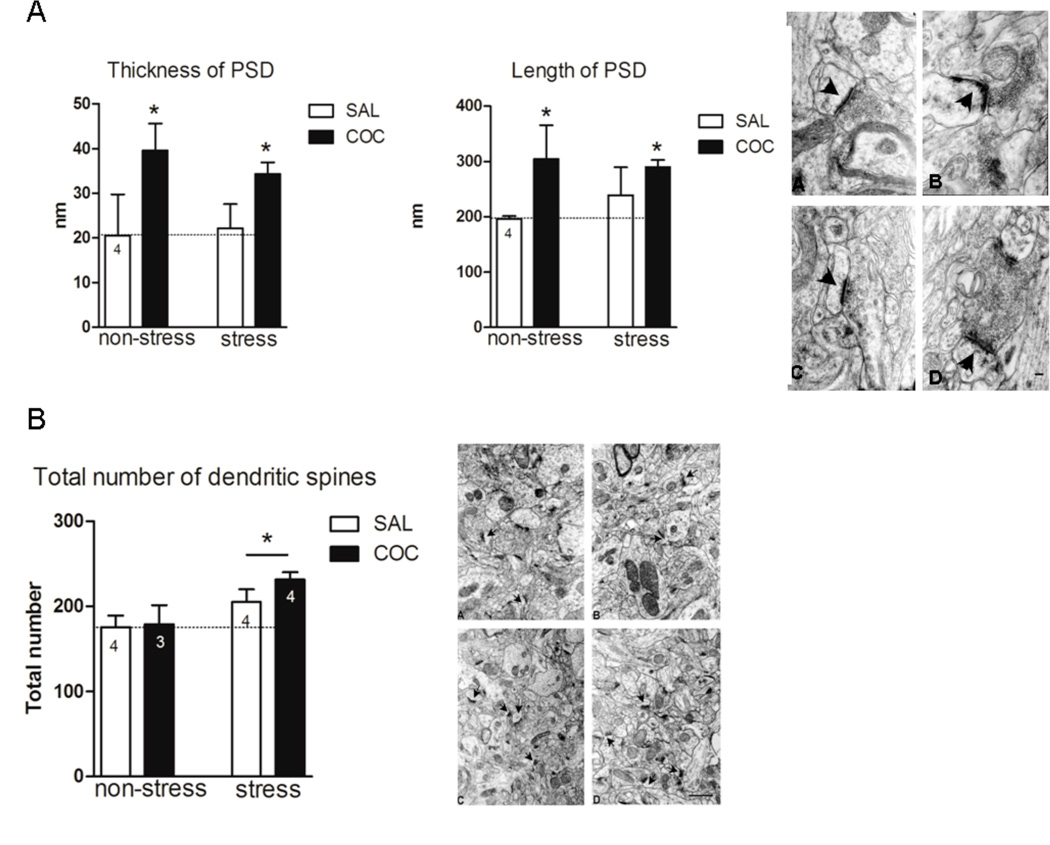
Figure 2.
Electron microscopy in the NAc. A, The length and thickness of PSD were measured in seven different fields for each animal under a magnification of 50,000 X. The length and thickness of PSD are expressed in nm as mean ± SEM. The results were analyzed by a two-way ANOVA (stress × drug) test. PSD length, stress F(1,14)=4.38, p=0.05; drug F(1,14)=20.25, p<0.001. *denotes p<0.05 compared to NS/SAL. PSD thickness, stress F(1,10)=5.20, p=0.045; drug F(1,10)=5.79, p=0.036. * p<0.05 compared to NS/SAL. The electron microphotograph depicted in A, shows the ultrastructure of PSD (arrows) of the NAc in all experimental conditions: (A) NS/SAL, (B) NS/COC, (C) S/SAL, (D) S/COC. Scale bar represents 100 nm. B, Dendritic spines were counted in forty different fields for each animal under a primary magnification of 12,500 X. Total number of dendritic spines is expressed as mean ± SEM. The electron microphotograph depicted in B shows the ultrastructure of the NAc neuropil in all experimental conditions: (A) NS/SAL, (B) NS/COC, (C) S/SAL, (D) S/COC. Scale bar represents 1 µm.
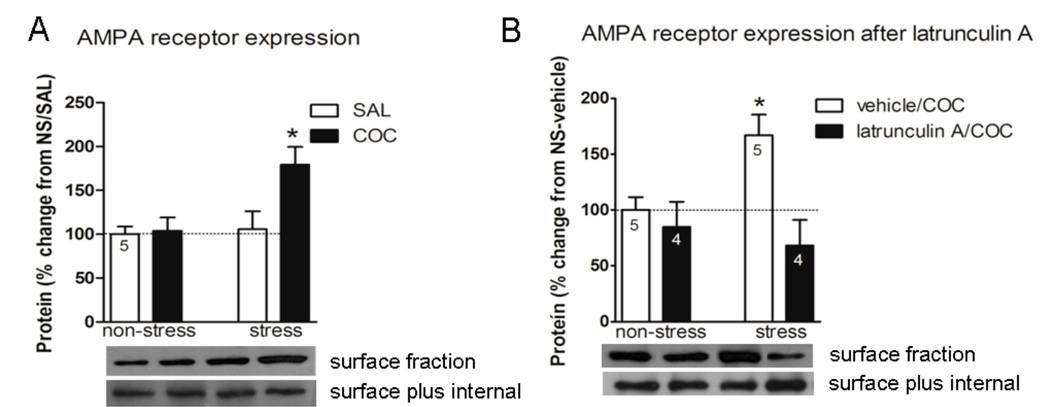
Figure 4.
GluR1 expression in the NAc. A, Three weeks after discontinuing repeated stress, rats were injected i.p. with saline, or cocaine 30 mg/kg and sacrificed 45 min later and the expression of the AMPAR subunit GluR1 was examined. B, Inhibiting actin cycling in the NAc prevents stress/cocaine induced elevation in AMPAR. Three weeks after discontinuing repeated stress, the animals were injected with latrunculin A (0.5 µg) or vehicle intra-NAc and 5 min later all animals were administered cocaine 30 mg/kg i.p. and sacrificed 45 min later and the expression of AMPAR was examined. Data is expressed as mean ± SEM of the number of determinations depicted in each graphic. The results were analyzed by a two-way ANOVA (stress × drug) and a Tukey´s test as a post hoc comparison when a significant interaction was present. A, stress × drug F(1,16)=4.25, p=0.05. * p<0.05 compared to all the remaining groups. B, stress × drug F(1,14)=4.77, p=0.046. * p<0.05 compared to stress-latrunculin A-cocaine and non-stress-latrunculin A-cocaine groups.
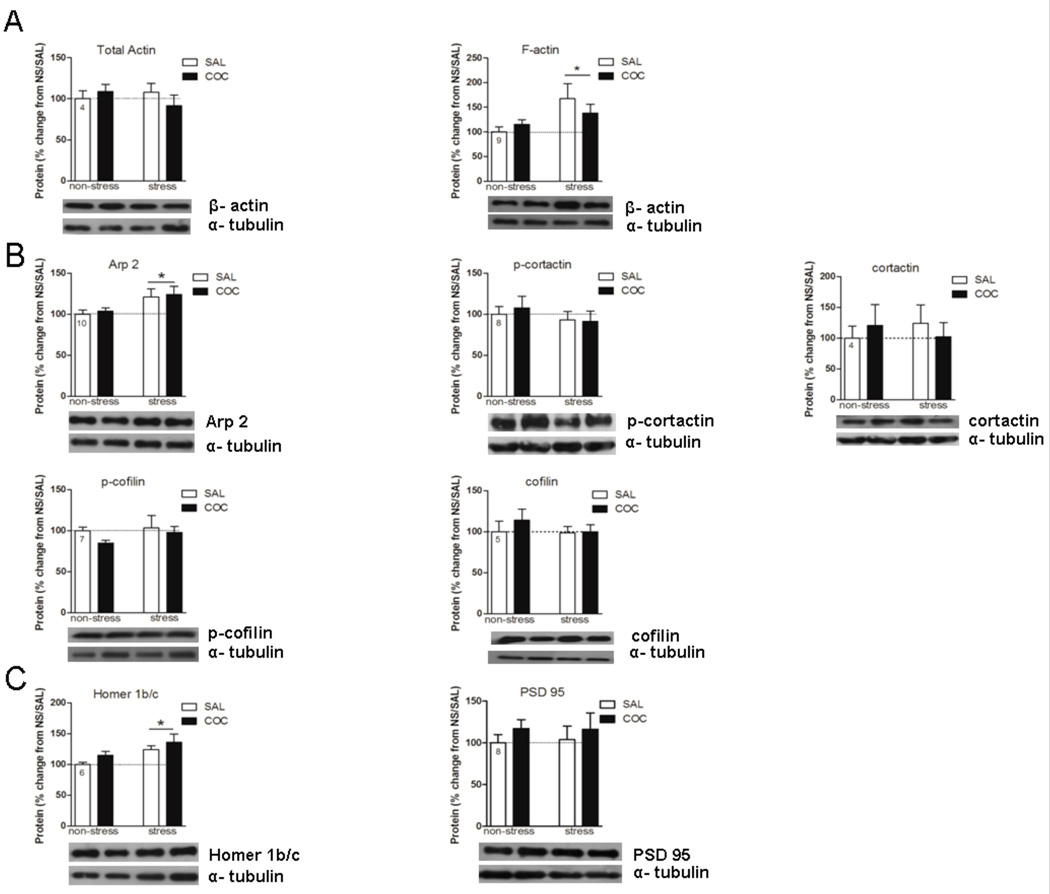
Figure 6.
Effects of repeated stress and cocaine on levels of F-actin, ABPs (actin-binding proteins) and PSD proteins in the Pfc. Data are expressed as mean ± SEM of the number of determinations depicted in each graphic. The results were analyzed by a two-way ANOVA (stress × drug) and a Tukey’s test as a post hoc comparison when a significant interaction was present. A, F-actin, stress F(1,32)=5.96, p=0.020. B, Arp 2, stress F(1,36)=7.23, p=0.010. C, Homer 1b/c, stress F(1,20)=7.41, p=0.013. *p<0.05 between the stress and the non-stress groups.
Figure 8.
Electron microscopy in the Pfc. A, The length and thickness of PSD were measured in seven different fields for each animal under a magnification of 50,000 X. Length and thickness of PSD are expressed in nm as mean ± SEM. The results were analyzed by a two-way ANOVA (stress × drug) test. PSD length, drug F(1,8)=8.10, p=0.022; PSD thickness, drug F(1,8)=38,32, p<0.001. *denotes significant difference between the cocaine and the saline groups. The electron microphotograph depicted in A shows the ultrastructure of PSD (arrows) of the Pfc in all experimental conditions: (A) NS/SAL, (B) NS/COC, (C) S/SAL, (D) S/COC. Scale bar represents 100 nm. B, Dendritic spines were counted in forty different fields for each animal under a primary magnification of 12,500 X. Total number of dendritic spines is expressed as mean ± SEM. B, stress F(1,11)=7.67, p=0.018. *p<0.05 between the stress and the non-stress groups. The electron microphotograph depicted in B shows the ultrastructure of the Pfc neuropil in all experimental conditions: (A) NS/SAL, (B) NS/COC, (C) S/SAL, (D) S/COC. Scale bar represents 1 µm.
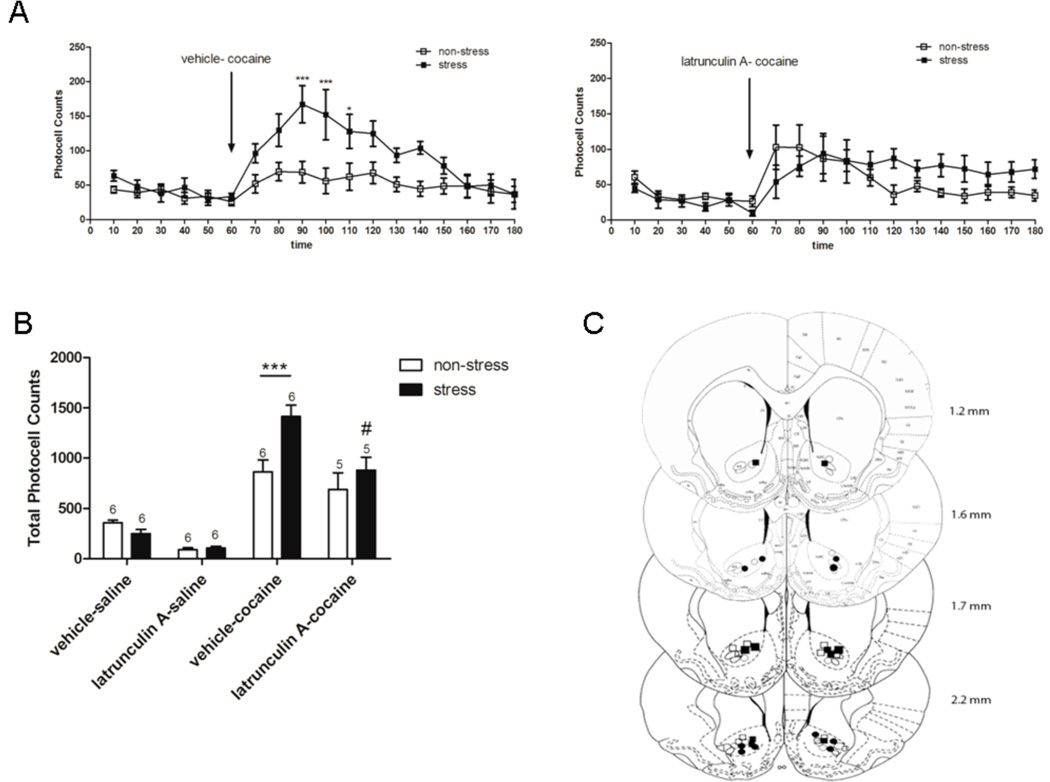
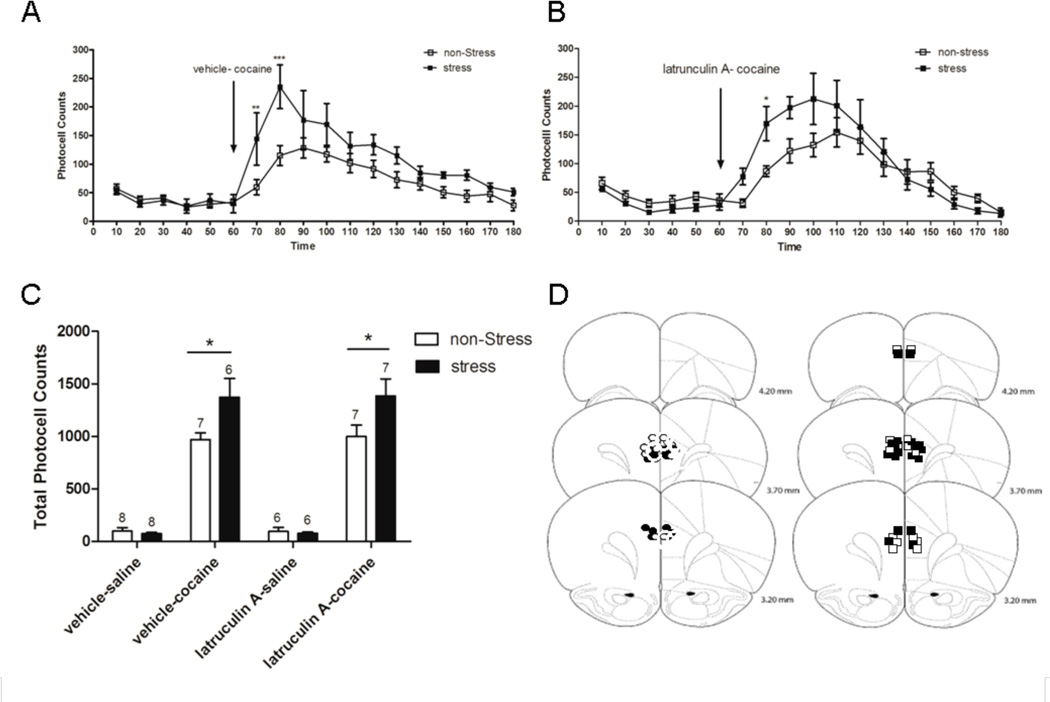
Figure 3.
Locomotor activity after cocaine (15 mg/kg i.p.) in rats pretreated with latrunculin A (0.5 µg) or vehicle (DMSO 1 %) into the NAc, 3 weeks after repeated immobilization stress. After 1 h habituation to test cages, the animals were injected with latrunculin A (n=6/group) or vehicle (n=7/group) intra-accumbens and 5 min later with saline or cocaine i.p. after which locomotor activity was monitored for 2 h. Values represent the mean ± SEM of horizontal photocell counts over 10 min periods. Total photocell counts over 120 min were shown as mean ± SEM. A, Time-course of locomotor activity was analyzed by a two-way ANOVA (non-stress/stress × latrunculin A/vehicle) with repeated measures over time: time F(17,374)=21.41, p<0.001; stress F(1,374)=18.05, p<0.001; stress × treatment F(1,22)=8.57, p=0.008; stress × treatment × time F(17,374)=6.03, p=0.008. * p<0.05 compared with all the remaining groups (non-stress-vehicle, non-stress-latrunculin A and stress-latrunculin A). # p<0.05 compared with non-stress-vehicle group. Comparisons between groups were at the same time point by the Tukey´s test. Arrow indicates 15 mg/kg cocaine i.p. injection. B, Total horizontal photocell counts 120 min after the cocaine injection was analyzed by a two-way ANOVA (non-stress/stress × latrunculin A/vehicle): stress F(1,22)=19.77, p<0.001; stress × treatment F(1,22)=7.36, p=0.013. * p<0.05 compared with all the remaining groups after cocaine injection. C, Location of the microinjection cannula tips in the NAc of rats included in the data analyses. The line drawings are from Paxinos and Watson (Paxinos & Watson, 2009). Numbers to the right indicate millimeters from bregma. Symbols represent the different groups: ○ (non-stress + latrunculin A intra-accumbens); ● (stress + latrunculin A intra-accumbens); □ (non-stress + vehicle intra-accumbens); ■ (stress + vehicle intra-accumbens).
Figure 7.
Locomotor activity after to cocaine (15 mg/kg i.p.) in rats pretreated with latrunculin A (0.5 µg) or vehicle (DMSO 1 %) in the Pfc, 3 weeks after repeated immobilization stress. After 1 h habituation to test cages, the animals were injected with latrunculin A (n=6–7/group) or vehicle (n=7–9/group) intra-prefrontal cortex and 5 min later with saline or cocaine i.p. after which locomotor activity was monitored for 2 h. Values represent the means ± SEM of horizontal photocell counts over 10 min periods. Total photocell counts over 120 min are shown as mean ± SEM. A-B, Time-course of locomotor activity was analyzed by a two-way ANOVA with repeated measures over time: A, stress F(1,238)=6.79, p=0.021; time F(17,238)=18.42, p<0.001; stress × time F(17,238)=2.32, p=0.003; B, time F(17,187)=19.98, p<0.001; stress × time F(17,187)=3.41, p<0.001. * p<0.05 compared with non-stress-vehicle and non-stress-latrunculin A respectively. Comparisons between groups were at the same time point by the Tukey´s test. Arrow indicates 15 mg/kg cocaine i.p. injection. C, Total horizontal photocell counts 120 min after the cocaine injection was analyzed by a two-way ANOVA (non-stress/stress × latrunculin A/vehicle): stress F(1,25)=9.44, p=0.005. * p<0.05 compared with non-stress groups after cocaine injection. D, Location of the microinjection cannula tips in the Pfc of rats included in the data analyses. The line drawings are from Paxinos and Watson (Paxinos & Watson, 2009). Numbers to the right indicate millimeters from bregma. Symbols represent the differents groups: ○ (non-stress + latrunculin A intraprefrontal cortex); ● (stress + latrunculin A intra-prefrontal cortex); □ (non-stress + vehicle intraprefrontal cortex); ■ (stress + vehicle intra-prefrontal cortex).
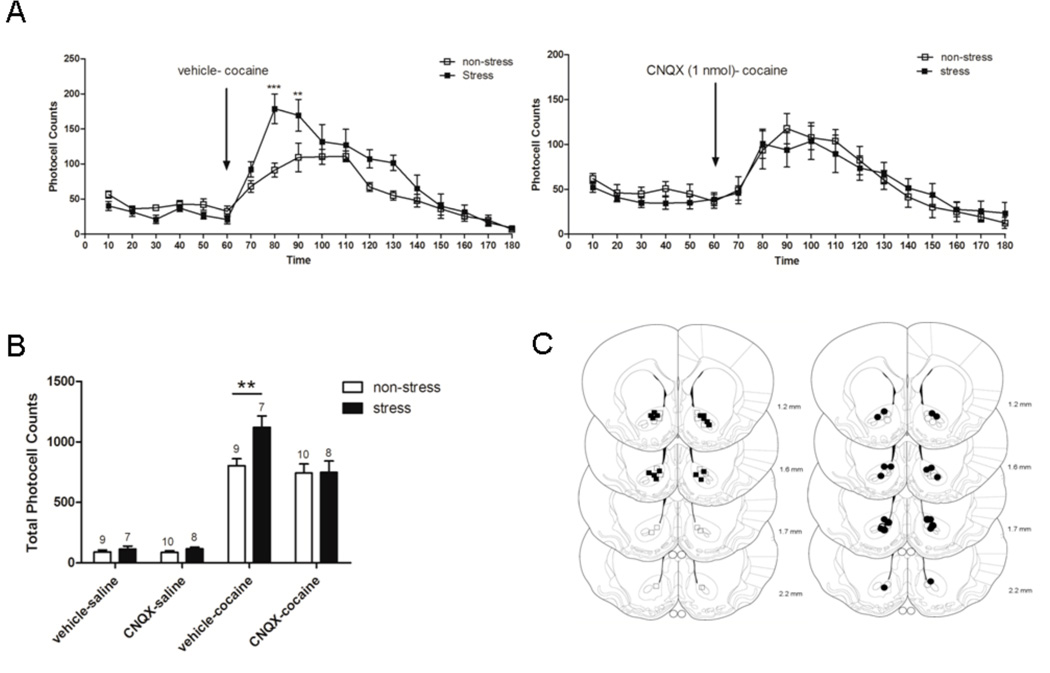
Figure 5.
Locomotor activity after cocaine (15 mg/kg i.p.) in rats pretreated with CNQX (1 nmol) or vehicle (DMSO 10 %) in the NAc, 3 weeks after repeated immobilization stress. After 1 h habituation to test cages, the animals were injected into the NAc with CNQX (n=9–10/group) or vehicle (n=7–8/group) and 5 min later with saline i.p. or cocaine i.p., after which locomotor activity was monitored for 2 h. Values represent the means ± SEM of horizontal photocell counts over 10 min periods. Total photocell counts over 120 min were shown as means ± SEM. A, Time-course of locomotor activity was analyzed by a two-way ANOVA (non-stress/stress × latrunculin A/vehicle) with repeated measures over time: time F(17,510)=40.01, p<0.001; stress × time F(17,510)=2.08, p=0.007; stress × treatment F(1,30)=5.19, p=0.030; stress × treatment × time F(17,510)=2.94, p<0.001. * indicates p<0.05 compared with all the remaining groups (non-stress-vehicle, non-stress-CNQX and stress-CNQX). # p<0.05 compared with stress-CNQX. Comparisons between groups were at the same time point by the Tukey´s test. Arrow indicates 15 mg/kg cocaine i.p. injection. B, Total horizontal photocell counts 120 min after the cocaine injection was analyzed by a two-way ANOVA (non-stress/stress × CNQX/vehicle): treatment F(1,30)=8.36, p=0.007; treatment × stress F(1,30)=7.98, p=0.008. * p<0.05 compared with all the remaining groups after cocaine injection. C, Location of the microinjection cannula tips in the NAc of rats included in the data analyses. The line drawings are from Paxinos and Watson (Paxinos & Watson, 2009). Numbers to the right indicate millimeters from bregma. Symbols represent the differents group: ○ (non-stress + CNQX 1 nmol intra-accumbens); ● (stress + CNQX 1nmol intra-accumbens); □ (non-stress + vehicle intra-accumbens); ■ (stress + vehicle intra-accumbens).
Results
Repeated Immobilization Stress and Cocaine Modify Actin Dynamics
A decrease in F-actin levels was observed in the NAc 21 days after the repeated stress exposure. Acute cocaine administration restored F-actin values to the levels observed in non-stress animals while the levels of total actin were not altered in any treatment group (Fig. 1B, stress×drug F(1,28)=6.54, p=0.016). Tukey´s post hoc test indicated that stress-saline group shows a significant decrease of F-actin values with regard to those observed in non-stress-saline group (p<0.05). The stress-induced decrease in F-actin levels may arise from a pronounced decrease in p-cofilin in the stress-saline compared to the non-stress-saline group (Fig. 1C, stress F(1,24)=4.77, p=0.039; drug F(1,24)=5.12, p=0.032) (Ono, 2003). However, the decrease in p-cofilin levels after cocaine in the non-stress group was not associated with a decrease in F-actin levels, whereas in the stress group, the cocaine injection restored F-actin to control levels without changing the stress-induced decrease in p-cofilin. This could reflect the action of active cofilin to increase filament branching, thereby increasing the amount of F-actin (Ono, 2003). Alternatively, other ABPs in addition to p-cofilin may contribute to the normalization of F-actin levels. Along these lines, cortactin is known to activate the Arp2/3 complex that also promotes the branching of new actin filaments from F-actin, and this action of cortactin is inhibited by Src-mediated tyrosine phosphorylation (Pollard & Borisy, 2003; Lua & Low, 2005). Thus, the cocaine-induced reduction of p-cortactin in stress-pretreated subjects may contribute to the restoration of F-actin and is consistent with the cocaine-induced increase in actin polymerization in prior repeated stress animals. The total tissue content of neither cofilin nor cortactin was altered (Fig. 1C, stress×drug F(1,22)=4.63, p=0.042). Tukey´s post hoc test indicates that the p-cortactin decrease in the stress-cocaine group was significantly different compared to all the remaining groups (p<0.05). PSD-95, one marker protein of postsynaptic density was unchanged after repeated stress or cocaine challenge (Fig. 1D). Another marker protein for the PSD, Homer 1b/c. Although Homer1b/c is reduced in the NAc following withdrawal from chronic cocaine (Szumlinski et al., 2004), it was not altered after prior repeated stress or cocaine challenge (Fig. 1D).
Cocaine Increases the PSD but not the Total Number of Dendritic Spines in NAc after Prior Repeated Stress
Figure 2 shows the results of an electron micrographic analysis of the number of dendritic spines and the size of PSD in the NAc. Twenty-one days after repeated inmobilization stress, an increase in the length and thickness of PSD in the NAc was observed following the cocaine injection (Fig. 2A, PSD length, stress F(1,14)=4.38, p=0.05; drug F(1,14)=20.25, p<0.001; PSD thickness, stress F(1,10)=5.20, p=0.045; drug F(1,10)=5.79, p=0.036). Fig. 2B, shows that the total number of dendritic spines in the NAc were not modified in animals administered either saline or cocaine following prior repeated stress. The lack of consistency between F-actin and dendritic spines density has been observed following other treatments, such as chronic morphine, which increased F-actin (Toda et al., 2006) and decreased the number of dendritic spines (Robinson & Kolb, 2004), and changes in F-actin may be more closely related to spine head diameter (Shen et al., 2009).
Inhibiting Actin Polymerization in the NAc Prevents Stress Cross-Sensitization with Cocaine
We have previously demonstrated that repeated stress induces lasting cross-sensitization of the locomotor response elicited by an acute cocaine (15 mg/kg i.p.) (Kalivas & Stewart, 1991; Sorg & Kalivas, 1991) or d-amphetamine injection (Pacchioni et al., 2007). Latrunculin A binds to G-actin thereby preventing its polymerization into F-actin (Morton et al., 2000), and the expression of cross-sensitization between repeated stress pretreatment and cocaine was prevented by intra-accumbens latrunculin A (0.5 µg/µl) administered 5 min before cocaine. Latrunculin A did not alter the motor response elicited by acute saline in either prior repeated stress or control animals, nor did it alter the motor response elicited by acute cocaine in control animals (Fig. 3A, time F(17,374)=21.41, p<0.001; stress F(1,374)=18.05, p<0.001; stress×treatment F(1,22)=8.57, p=0.008; stress×treatment×time F(17,374)=6.03, p=0.008). Tukey`s post-hoc comparisons indicated that the stress-vehicle group shows significantly higher photocell counts at 90 and 100 min compared with the stress-latrunculin A and all the remaining groups. These comparisons also showed that counts at 70 and 80 min in the stress-vehicle group were significantly higher than those of the non-stress-vehicle group. The total photocell counts over 120 min after cocaine injection also showed the higher response of the stress-vehicle group compared to all the remaining groups (Fig. 3B, stress F(1,22)=19.77, p<0.001; stress×treatment F(1,22)=7.36, p=0.013). Tukey`s post-hoc comparisons indicated that a significant difference (p<0.05) in the total cumulative counts was observed between the stress-vehicle group and all the remaining groups after cocaine injection.
Prior Repeated Stress and Cocaine-Induced Increase in the Surface Expression of GluR1 is Prevented by Inhibiting the Actin Polymerization in the NAc
Given that the size of PSDs is positively correlated with spine head diameter, and since spines with large PSDs exhibit higher levels of AMPAR immunoreactivity than spines with smaller PSDs (Harris & Stevens, 1989; Matsuzaki et al., 2001; Nakagawa et al., 2005), it is likely that, in the Nac of the stress/cocaine group, large spines (i.e. with greater size of PSDs) would also express a greater number of glutamate receptors. Consistently with the increase in PSD observed in prior repeated stress animals after acute cocaine (Fig. 2A) (Ehrlich & Malinow, 2004; Gerges et al., 2004), surface expression of the AMPA receptor subunit GluR1 was higher in prior repeated stress animals administered cocaine than in the remaining groups (Fig. 4A, stress×drug F(1,16)=4.25, p=0.05). Tukey´s post hoc test indicated a significant difference between the stress-cocaine and the remaining groups (p<0.05). Since PSD-95, which may help anchor GluR1-containing AMPAR in the membrane (Ehrlich & Malinow, 2004; Gerges et al., 2004) was unchanged, it is likely that other proteins such as stargazin, which bind both AMPAR and PSD-95 could be participating, as has been proposed in hippocampus (Schnell et al., 2002; Santos et al., 2009). Latrunculin A in the NAc also reversed the stress/cocaine-induced increase in surface expression of GluR1 (Fig. 4B, stress×treatment F(1,14)=4.77, p=0.046). Tukey´s post hoc test revealed that the stress-vehicle-cocaine group had significantly increased GluR1 compared to the stress-latrunculin A-cocaine and non-stress-latrunculin A-cocaine groups (p<0.05), indicating a potential role for actin dynamics in the increased expression of GluR1 accompanying the expression of cocaine cross-sensitization. Interestingly, latrunculin A had no effect on GluR1 surface expression in control animals, further supporting the possibility of a causal linkage between the increased GluR1, actin dynamics, and cocaine-induced locomotion in the stress/cocaine group.
Blockade of AMPAR within the NAc Prevents the Expression of Stress Cross-Sensitization with Cocaine
Akin to latrunculin A, pretreatment with CNQX (1 nmol) into the NAc significantly abrogated the sensitized response to cocaine following stress, without attenuating the locomotor response to acute cocaine in non-stressed animals. The locomotor enhancement observed after cocaine injection into the repeated stress group was prevented by a CNQX pretreatment (Fig. 5A, time F(17,510)=40.01, p<0.001; stress×time F(17,510)=2.08, p=0.007; stress×treatment F(1,30)=5.19, p=0.030; stress×treatment×time F(17,510)=2.94, p<0.001). Tukey´s post hoc test indicated that photocell counts at 80 min in the stress-vehicle group were significantly higher than those observed in the stress-CNQX and all the non-stress groups, and that counts at 90 min in the stress-vehicle group were significantly higher than that of the stress-CNQX group. Consistently, the total photocell counts over 120 min after cocaine challenge in stress-vehicle was significantly increased compared to the stress-CNQX and all non-stress groups (Fig. 5B, treatment F(1,30)=8.36, p=0.007; treatment×stress F(1,30)=7.98, p=0.008). Tukey´s post hoc test comparisons indicated that the total cumulative counts in the stress-vehicle group was significantly increased with regard to the stress-CNQX and all non-stress-vehicle groups. This finding is consistent with the pharmacological study of (Pierce et al., 1996) who showed that the microinjection of CNQX into the NAc core abolished the augmented motor response to a cocaine challenge in sensitized rats, but was without effect on cocaine-induced motor activity in nonsensitized animals.
Actin Polymerization in the Pfc is Promoted by Stress, but Does not Regulate Cocaine Motor Activity
Twenty-one days after repeated immobilization stress exposure, an increase in F-actin levels in the Pfc was observed either after saline or cocaine, meanwhile the levels of total actin were not altered in any treatment group (Fig. 6A, stress F(1,32)=5.96, p=0.020). Consistent with the increase in F-actin, an increase in Arp2, which promotes actin filament branching and elongation (May, 2001; Pollard, 2007), was observed in the stress group compared to the non-stress group (Fig. 6B, stress F(1,36)=7.23, p=0.010). While no differences in PSD-95 were measured between any treatment groups in the Pfc, repeated pre-stress elicited an increase in Homer 1b/c regardless of whether the animals were administered cocaine or saline (Fig. 6C, stress F(1,20)=7.41, p=0.013). None of the other actin-binding proteins measured were significantly altered in the Pfc in any treatment group (Fig. 6).
Pretreatment with latrunculin A into the Pfc before a cocaine challenge did not alter the motor response in prior repeated stress animals. Latrunculin A did not modify the motor response elicited by acute saline or cocaine in control animals nor did it influence that after saline in repeated pre-stress animals. The locomotor enhancement of the behavioral response to cocaine observed in stress group was not modified by latrunculin A treatment (Fig. 7A, stress F(1,238)=6.79, p=0.021; time F(17,238)=18.42, p<0.001; stress×time F(17,238)=2.32, p=0.003; Fig. 7B, time F(17,187)=19.98, p<0.001; stress×time F(17,187)=3.42, p<0.001). Tukey´s post-hoc comparisons indicated that the stress-vehicle group shows significantly higher photocell counts at 80 min compared with the non-stress-vehicle group. The enhanced motor response to cocaine at 70, 80 and 90 min in the stress-latrunculin A group was significantly higher than that in the non-stress-latrunculin A group. The total photocell counts over 120 min after cocaine injection also showed the higher response of the stress-vehicle and stress-latrunculin A groups compared with the nonstress- vehicle and non-stress-latrunculin A, respectively (Fig. 7C, stress F(1,25)=9.44, p=0.005).
Cocaine Increases the PSD but Does not Modify the Stress-Induced Increase in the Total Number of Dendritic Spines in the Pfc
Figure 8 shows the results of an electron micrographic analysis of the number of spines and the size of the PSD in the Pfc. Acute cocaine increased PSD thickness and length regardless of the pretreatment group (Fig. 8A, PSD length, drug F(1,8)=8.10, p=0.022; PSD thickness, drug F(1,8)=38,32, p<0.001). The density of spines was increased only in prior repeated stress rats regardless of the administration of acute saline or cocaine (Fig. 8B, stress F(1,11)=7.67, p=0.018). Thus, in the Pfc, the change in spine density seems to be associated with homeostatic changes in the stress group, whereas those in PSD are more related with the acute effect of cocaine.
Prior Repeated Stress and Cocaine Do not Alter Actin Dynamics in the Striatum
Three weeks after the repeated immobilization stress, no influence on F-actin or p-cofilin levels was elicited in the striatum after either a saline or cocaine injection (data not shown). This agrees with studies showing no influence of repeated cocaine withdrawal on the actin cytoskeleton in striatum (Toda et al., 2006).
Discussion
This study identified neuroadaptations in the NAc that contribute to the cross-sensitization between repeated stress and acute cocaine, including increases in the PSD size, actin dynamics and AMPAR surface expression. We observed that repeated stress exposure was associated with long-term changes in proteins that regulate the actin cytoskeleton in the NAc, and that disrupting actin dynamics with latrunculin A prevented the expression of cocaine cross-sensitization with stress. Similar changes and a role for actin dynamics has been shown for sensitization to daily cocaine injections in rats expressing behavioral sensitization after withdrawal from daily cocaine injections (Toda et al., 2006; Toda et al., 2010), indicating that cocaine sensitization induced by repeated stress or cocaine may share this underlying adaptation. Moreover, the stress-related changes in actin dynamics and cross-sensitization were associated with GluR1 surface expression in the NAc. Thus, only in prior repeated stress rats did acute cocaine increase GluR1 surface expression, and the sensitized motor response in this treatment group was abolished by intra-accumbens blockade of AMPAR with CNQX. A similar adaptation in AMPAR has been previously reported after cocaine withdrawal, and is thought to contribute to the expression of behavioral sensitization after daily cocaine (Boudreau & Wolf, 2005; Ghasemzadeh et al., 2009; Schumann & Yaka, 2009). Finally, since drugs and stress modulate the density and morphology of spines (Kolb & Whishaw, 1998; Robinson & Kolb, 2004; Shen et al., 2009; Christoffel et al., 2011), we measured spine density and the length and thickness of the PSD. Although spine density was not affected by any treatment, the size of PSD was increased only in the prior repeated stress animals injected acutely with cocaine. Taken together, the sensitized behavioral response to acute cocaine observed in the prior repeated stress rats was associated with increased actin dynamics, GluR1 surface expression and PSD size in the NAc and the causal nature of this association is supported by the fact that inhibiting actin dynamics or AMPAR in the NAc prevents the sensitized motor response.
Effects of Stress and Cocaine on ABPs that Regulate Actin Dynamics, Surface GluR1, PSD size and Dendritic Spines in the NAc
Binding of the ADF/cofilin family of proteins to F-actin promotes filament disassembly, and actin binding by cofilin is terminated by phosphorylation (Ono, 2003). Thus, a decrease in p-cofilin increases active cofilin and may increase F-actin depolymerization, thereby reducing F-actin in the prior repeated stress. Chen et al., (2008) also reported a decrease in p-cofilin in the hippocampus following stress. Decreased cofilin phosphorylation increases cofilin binding affinity for F-actin, and the dissociation rate constant for actin subunits from the filament’s pointed end (Bamburg, 1999). Cofilin also promotes actin nucleation due to the cofilin-severed F-actin fragments acting as preferred substrates from which Arp2/3 builds actin networks (Van Troys et al., 2008). In addition to reduced p-cofilin, we reported a decrease in p-cortactin after cocaine in animals pretreated with repeated stress. Thus, the increased availability of G-actin by cofilin-mediated depolymerization could be preferentially routed to form lamellipodia-like protein complexes. This might lead to enhanced levels of cortactin and, as a consequence, the Arp2/3 protein would be activated, promoting nucleation and polymerization in the branched actin networks (Lua & Low, 2005). The decrease in both p-cofilin and p-cortactin is consistent with a simultaneous enhancement in actin polymerization and depolymerization, which is indicative of increased actin cycling and changes in dendritic spine morphology (Meng et al., 2003; Ono, 2003; Pollard & Borisy, 2003; Ghosh et al., 2004), as previously demonstrated after withdrawal from chronic cocaine (Toda et al., 2006).
The increase in AMPAR found after cocaine in prior repeated stress animals parallels the alterations in actin dynamics, suggesting that both events may be related. Although our data do not address the precise role for cofilin and cortactin mediated actin dynamics in GluR1 trafficking, the reduction in GluR1 surface expression by latrunculin A appears to rule out a simply passive role for the actin dynamics. While cofilin can break down the existing actin filaments, it can also generate new barbed ends for further actin polymerization (Van Troys et al., 2008). It is thus conceivable that cofilin and cortactin may produce two coordinated effects on the actin network for AMPAR insertion: cofilin severing activity increases the number of preferred ends for Arp2/3 nucleation (Ichetovkin et al., 2002) and cortactin can bind to and activate the Arp2/3 complex favouring the stabilization of the actin network (Pontrello & Ethell, 2009). As shown by Allison et al., (1998) the enhanced actin polymerization and stabilized actin filaments (F-actin) favour surface expression of AMPARs. Furthermore, recent evidence shows that elevated ADF/cofilin potentiates, while ADF/cofilin inhibition abolishes AMPAR insertion (Gu et al., 2010). Although it is important that future studies untangle the contributions of depolymerization and polymerization to the changes in AMPAR, this study provides a platform from which their contribution to stress- and drug-induced sensitization to cocaine can be further investigated.
It is generally thought that receptor trafficking during plasticity is coupled to morphological changes in postsynaptic spines. Accordingly, we found that cocaine induced an increase in AMPAR surface expression as well as in the size of PSD in prior repeated stress animals, without changes in F-actin levels and the total number of dendritic spines. Thus, akin to other studies, changes in actin may be accompanied by changes in spine morphology without significantly altering the number of spines (Calabrese et al., 2006; Shen et al., 2009). Consistent with the apparent association we identified, the size of the PSD is correlated with spine head diameter (Harris & Stevens, 1989; Kasai et al., 2003), and spines with large heads express a greater density of AMPAR (Kasai et al., 2003; Matsuzaki et al., 2001). Together, these studies are consistent with the possibility that the increase in the size of PSD and number of AMPAR observed in the stress-cocaine group may contribute to the expression of stress/cocaine cross-sensitization.
The pioneering studies showing that corticosterone secretion is one of the mechanisms underlying stress-induced cross-sensitization to amphetamine, morphine and cocaine, may also points to a potential mechanism whereby stress may elicit changes in actin dynamics and spine morphology (Deroche et al., 1992; Deroche et al., 1993; Deroche et al., 1995; Rouge-Pont et al., 1995; Rouge-Pont et al., 1998; Prasad et al., 1998). It has been suggested that the increase in glucocorticoid hormones, through action on mesolimbic dopamine neurons, underlies the increased vulnerability to drug abuse (Marinelli & Piazza, 2002; de Jong & de Kloet, 2004), and cold water stress exposure can enhance synaptic strength in NAc MSNs or midbrain dopamine neurons via a glucocorticoid-dependent mechanism (Saal et al., 2003; Campioni et al., 2009). Also, selective deletion of Nr3c1 (the glucocorticoid receptor gene) in mouse dopaminoceptive neurons expressing D1 dopamine receptors decreased the motivation of mice to self-administer cocaine (Ambroggi et al., 2009). Recent studies also reveal that corticosterone plays a role in generating dendritic spine plasticity in the NAc (Morales-Medina et al., 2009) and hippocampus (Liston & Gan, 2011), as well as in increasing AMPAR surface mobility and synaptic surface content in the latter (Martin et al., 2009; Conboy & Sandi, 2010; Groc et al., 2008). In addition to corticosterone, several lines of evidence indicate a critical role for corticotropin releasing factor (CRF) actions on the dopamine system in stress-related behaviors. Neurons containing CRF project to and make synapses within the VTA (Tagliaferro & Morales, 2008), and injection of CRF directly into the VTA increases locomotor activity (Kalivas et al., 1987), while injection of CRF antagonists inhibits stress-induced reinstatement of cocaine-seeking (Wang et al., 2005; Wang et al., 2007) as well as cross-sensitization to psychostimulants (Cole et al., 1990; Boyson et al., 2011). Moreover, electrophysiological recordings have shown that CRF regulates excitability of VTA dopamine neurons (Korotkova et al., 2006; Wanat et al., 2008) and synaptic inputs onto these neurons (Ungless et al., 2003). In addition, it was shown in non-dopaminergic areas that CRF modifies the actin cytoskeleton (Swinny & Valentino, 2006). Taken together these data point to CRF and corticosterone secretion by stress as important potential mechanisms in the stress-induced increases in actin cycling we have identified that predispose animals to the motor stimulant effects of psychostimulants.
Inhibiting Actin Polymerization with Latrunculin A in the NAc Prevents Stress Cross-Sensitization with Cocaine and Stress-induced Elevation in GluR1
Consistent with a causal influence of altered actin dynamics and GluR1, treatment of the NAc with latrunculin reversed both the expression of stress-induced cross-sensitization with cocaine and the elevation in surface expression of GluR1. Likewise, both AMPAR-mediated currents following repetitive release of glutamate and the density of AMPAR in hippocampal neurons are reduced by latrunculin (Matsuzaki et al., 2004; Allison et al., 1998). Moreover, blocking AMPAR with CNQX inhibited the expression of cross-sensitization between stress and cocaine. Likewise, microdialysis data from our laboratory indicates that glutamate is involved in the long-lasting sensitized response to psychostimulants following stress (Pacchioni et al., 2007). These observations are consistent with previous studies showing an involvement of AMPAR in the cocaine-induced sensitization (Pierce et al., 1996), an increased AMPAR at twenty-one days of cocaine withdrawal (Boudreau & Wolf, 2005; Ghasemzadeh et al., 2009; Schumann & Yaka, 2009), and studies suggesting that increased postsynaptic responsiveness of excitatory synapses in the NAc plays an important role in the behavioral effects of chronic cocaine (Kourrich et al., 2007; Famous et al., 2008). Furthermore, the inhibition of calcium permeable AMPAR, a hallmark of late-stage cocaine-evoked synaptic plasticity in the NAc (Conrad et al., 2008), and the viral expression of a peptide that impairs GluR1 trafficking (Anderson et al., 2008) reduce cue-induced cocaine seeking and cocaine-primed reinstatement, respectively. Nonetheless, although the effect of latrunculin on GluR1 levels argues for an action in the postsynapse, latrunculin will inhibit actin cycling in all cell types and in all regions of the cell where actin is present. Thus, actin in other cellular compartments may contribute to the behavioral effects. In addition, it should be noted that animals can demonstrate sensitized behaviors after early withdrawal but do not express AMPAR upregulation (Boudreau & Wolf, 2005; Ghasemzadeh et al., 2009; Schumann & Yaka, 2009), and that GluR1 surface expression in the NAc is not altered 30 min after cocaine challenge, even though the rats expressed locomotor sensitization (Boudreau et al., 2007; Ferrario et al., 2011). Thus, although increased AMPAR is not the only mechanism underlying behavioral sensitization or cross-sensitization with cocaine, our current findings that CNQX and latrunculin A in the NAc prevented the expression of cross-sensitization between cocaine and stress supports the importance of both actin cycling and AM-PAR in stress-induced sensitized behaviors.
Effects of Prior Repeated Stress and Cocaine on the ABPs that Regulate Actin Cycling, PSD size and Dendritic Spines in the Pfc
F-actin levels, as well as those of the polymerizing protein Arp2 and the scaffolding protein Homer 1b/c, were increased in the Pfc of all the stress groups, while p-cofilin levels were not altered. The increase in Homer 1b/c levels in all the stress groups is in agreement with that observed in the Pfc from early stressed animals, submitted to a repeated restraint stress in adulthood as well as after a cocaine self-administration paradigm (Szumlinski et al., 2008). Usui et al., (2003) have shown long isoforms of Homer to be associated with the formation of synaptic contacts and the synaptic accumulation of F-actin at the PSD. The latter could be associated with increased F-actin and Arp2. Thus, the increase in Arp2 after prior repeated stress predicts an increase in actin filament branches and an increase in dendritic spine density (May, 2001; Pollard, 2007). There is evidence that after long immobilization stress, atrophy takes place in the dendrites and spines in the Pfc. However, 21 days after discontinuing stress, the atrophy rebounds, as reflected by an increase in dendritic length and density of spines (Seib & Wellman, 2003; Brown et al., 2005; Radley et al., 2008; Goldwater et al., 2009); which is in agreement with the observations in this study. Since an increase in the PSD (length and thickness) was observed after cocaine in both nonstress and stress groups, it is not likely that this strongly contributes to the expression of cross-sensitization between repeated stress and cocaine. The lack of influence of latrunculin A in Pfc on the sensitized response to cocaine also raises the possibility that prolonged immobilization stress may trigger homeostatic mechanisms in the Pfc that are unrelated to the expression of stress-induced cross-sensitization.
Summary
The main conclusion from our study is that a history of repeated stress alters the capacity of a subsequent cocaine injection to modulate dendritic spine morphology (size of PSD), actin dynamics and AMPAR expression in the NAc. Furthermore, the alterations in actin dynamics regulate AMPAR surface expression in the NAc after cocaine in the prior repeated stress group, which is a shared molecular mechanism between stress- and drug-induced sensitization to cocaine. Also, we found that actin dynamics and spine morphology changes in the Pfc following stress are not associated with the expression of cross-sensitization to cocaine. These are among the first findings to demonstrate a common cellular mechanism underpinning the expression of behavioral cross-sensitization between stress and cocaine. Given the well-established synergism between stress and cocaine addiction, these mechanisms pose common points of pharmacological intervention in treating cocaine addiction and its interactions with stressful life experiences.
Acknowledgements
This work was supported by grants from FONCyT, CONICET, SECyT and MinCyT (Argentina). The authors are grateful to Estela Salde and Lorena Mercado for her laboratory technical assistance and Joss Heywood for English technical assistance.
Abbreviations
- ABP
actin-binding protein
- AMPAR
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor
- CNQX
6-cyano-7-nitroquinoxaline-2,3-dione
- CRF
corticotropin releasing factor
- EM
electron microscopic
- NAc
nucleus accumbens
- Pfc
prefrontal cortex
- PSD
postsynaptic density
References
- Allison DW, Gelfand VI, Spector I, Craig AM. Role of actin in anchoring postsynaptic receptors in cultured hippocampal neurons: differential attachment of NMDA versus AMPA receptors. J Neurosci. 1998;18:2423–2436. doi: 10.1523/JNEUROSCI.18-07-02423.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambroggi F, Turiault M, Milet A, Deroche-Gamonet V, Parnaudeau S, Balado E, Barik J, van der Veen R, Maroteaux G, Lemberger T, Schutz G, Lazar M, Marinelli M, Piazza PV, Tronche F. Stress and addiction: glucocorticoid receptor in dopaminoceptive neurons facilitates cocaine seeking. Nat Neurosci. 2009;12:247–249. doi: 10.1038/nn.2282. [DOI] [PubMed] [Google Scholar]
- Anderson SM, Famous KR, Sadri-Vakili G, Kumaresan V, Schmidt HD, Bass CE, Terwilliger EF, Cha JH, Pierce RC. CaMKII: a biochemical bridge linking accumbens dopamine and glutamate systems in cocaine seeking. Nat Neurosci. 2008;11:344–353. doi: 10.1038/nn2054. [DOI] [PubMed] [Google Scholar]
- Bamburg JR. Proteins of the ADF/cofilin family: essential regulators of actin dynamics. Annu Rev Cell Dev Biol. 1999;15:185–230. doi: 10.1146/annurev.cellbio.15.1.185. [DOI] [PubMed] [Google Scholar]
- Bhatt DH, Zhang S, Gan WB. Dendritic spine dynamics. Annu Rev Physiol. 2009;71:261–282. doi: 10.1146/annurev.physiol.010908.163140. [DOI] [PubMed] [Google Scholar]
- Boudreau AC, Reimers JM, Milovanovic M, Wolf ME. Cell surface AMPA receptors in the rat nucleus accumbens increase during cocaine withdrawal but internalize after cocaine challenge in association with altered activation of mitogen-activated protein kinases. J Neurosci. 2007;27:10621–10635. doi: 10.1523/JNEUROSCI.2163-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau AC, Wolf ME. Behavioral sensitization to cocaine is associated with increased AMPA receptor surface expression in the nucleus accumbens. J Neurosci. 2005;25:9144–9151. doi: 10.1523/JNEUROSCI.2252-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyson CO, Miguel TT, Quadros IM, Debold JF, Miczek KA. Prevention of social stress-escalated cocaine self-administration by CRF-R1 antagonist in the rat VTA. Psychopharmacology (Berl) 2011;218:257–269. doi: 10.1007/s00213-011-2266-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady KT, Sinha R. Co-occurring mental and substance use disorders: the neurobiological effects of chronic stress. Am J Psychiatry. 2005;162:1483–1493. doi: 10.1176/appi.ajp.162.8.1483. [DOI] [PubMed] [Google Scholar]
- Brown SM, Henning S, Wellman CL. Mild, short-term stress alters dendritic morphology in rat medial prefrontal cortex. Cereb Cortex. 2005;15:1714–1722. doi: 10.1093/cercor/bhi048. [DOI] [PubMed] [Google Scholar]
- Calabrese B, Wilson MS, Halpain S. Development and regulation of dendritic spine synapses. Physiology (Bethesda) 2006;21:38–47. doi: 10.1152/physiol.00042.2005. [DOI] [PubMed] [Google Scholar]
- Campioni MR, Xu M, McGehee DS. Stress-induced changes in nucleus accumbens glutamate synaptic plasticity. J Neurophysiol. 2009;101:3192–3198. doi: 10.1152/jn.91111.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancela LM, Volosin M, Molina VA. Gangliosides attenuate stress-induced changes on body weight, motor activity and on the behavioral response to 5-methoxy-N,N-dimethyltryptamine. Brain Res Bull. 1996;40:105–110. doi: 10.1016/0361-9230(96)00040-8. [DOI] [PubMed] [Google Scholar]
- Carlisle HJ, Kennedy MB. Spine architecture and synaptic plasticity. Trends Neurosci. 2005;28:182–187. doi: 10.1016/j.tins.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Cingolani LA, Goda Y. Actin in action: the interplay between the actin cytoskeleton and synaptic efficacy. Nat Rev Neurosci. 2008;9:344–356. doi: 10.1038/nrn2373. [DOI] [PubMed] [Google Scholar]
- Cole BJ, Cador M, Stinus L, Rivier J, Vale W, Koob GF, Le Moal M. Central administration of a CRF antagonist blocks the development of stress-induced behavioral sensitization. Brain Res. 1990;512:343–346. doi: 10.1016/0006-8993(90)90646-S. [DOI] [PubMed] [Google Scholar]
- Conboy L, Sandi C. Stress at learning facilitates memory formation by regulating AMPA receptor trafficking through a glucocorticoid action. Neuropsychopharmacology. 2010;35:674–685. doi: 10.1038/npp.2009.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, Marinelli M, Wolf ME. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Dube CM, Rice CJ, Baram TZ. Rapid loss of dendritic spines after stress involves derangement of spine dynamics by corticotropin-releasing hormone. J Neurosci. 2008;28:2903–2911. doi: 10.1523/JNEUROSCI.0225-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffel DJ, Golden SA, Dumitriu D, Robison AJ, Janssen WG, Ahn HF, Krishnan V, Reyes CM, Han MH, Ables JL, Eisch AJ, Dietz DM, Ferguson D, Neve RL, Greengard P, Kim Y, Morrison JH, Russo SJ. IkappaB kinase regulates social defeat stress-induced synaptic and behavioral plasticity. J Neurosci. 2011;31:314–321. doi: 10.1523/JNEUROSCI.4763-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong IE, de Kloet ER. Glucocorticoids and vulnerability to psychostimulant drugs: toward substrate and mechanism. Ann N Y Acad Sci. 2004;1018:192–198. doi: 10.1196/annals.1296.022. [DOI] [PubMed] [Google Scholar]
- Deroche V, Marinelli M, Maccari S, Le Moal M, Simon H, Piazza PV. Stress-induced sensitization and glucocorticoids. I. Sensitization of dopamine-dependent locomotor effects of amphetamine and morphine depends on stress-induced corticosterone secretion. J Neurosci. 1995;15:7181–7188. doi: 10.1523/JNEUROSCI.15-11-07181.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deroche V, Piazza PV, Casolini P, Le Moal M, Simon H. Sensitization to the psychomotor effects of amphetamine and morphine induced by food restriction depends on corticosterone secretion. Brain Res. 1993;611:352–356. doi: 10.1016/0006-8993(93)90526-s. [DOI] [PubMed] [Google Scholar]
- Deroche V, Piazza PV, Casolini P, Maccari S, Le Moal M, Simon H. Stress-induced sensitization to amphetamine and morphine psychomotor effects depend on stress-induced corticosterone secretion. Brain Res. 1992;598:343–348. doi: 10.1016/0006-8993(92)90205-n. [DOI] [PubMed] [Google Scholar]
- dos Remedios CG, Chhabra D, Kekic M, Dedova IV, Tsubakihara M, Berry DA, Nosworthy NJ. Actin binding proteins: regulation of cytoskeletal microfilaments. Physiol Rev. 2003;83:433–473. doi: 10.1152/physrev.00026.2002. [DOI] [PubMed] [Google Scholar]
- Ehrlich I, Malinow R. Postsynaptic density 95 controls AMPA receptor incorporation during long-term potentiation and experience-driven synaptic plasticity. J Neurosci. 2004;24:916–927. doi: 10.1523/JNEUROSCI.4733-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famous KR, Kumaresan V, Sadri-Vakili G, Schmidt HD, Mierke DF, Cha JH, Pierce RC. Phosphorylation-dependent trafficking of GluR2-containing AMPA receptors in the nucleus accumbens plays a critical role in the reinstatement of cocaine seeking. J Neurosci. 2008;28:11061–11070. doi: 10.1523/JNEUROSCI.1221-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario CR, Li X, Wolf ME. Effects of acute cocaine or dopamine receptor agonists on AMPA receptor distribution in the rat nucleus accumbens. Synapse. 2011 doi: 10.1002/syn.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A, Sananbenesi F, Schrick C, Spiess J, Radulovic J. Distinct roles of hippocampal de novo protein synthesis and actin rearrangement in extinction of contextual fear. J Neurosci. 2004;24:1962–1966. doi: 10.1523/JNEUROSCI.5112-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerges NZ, Backos DS, Esteban JA. Local control of AMPA receptor trafficking at the postsynaptic terminal by a small GTPase of the Rab family. J Biol Chem. 2004;279:43870–43878. doi: 10.1074/jbc.M404982200. [DOI] [PubMed] [Google Scholar]
- Ghasemzadeh MB, Vasudevan P, Mueller C, Seubert C, Mantsch JR. Region specific alterations in glutamate receptor expression and subcellular distribution following extinction of cocaine self-administration. Brain Res. 2009 doi: 10.1016/j.brainres.2009.01.047. [DOI] [PubMed] [Google Scholar]
- Ghosh M, Song X, Mouneimne G, Sidani M, Lawrence DS, Condeelis JS. Cofilin promotes actin polymerization and defines the direction of cell motility. Science. 2004;304:743–746. doi: 10.1126/science.1094561. [DOI] [PubMed] [Google Scholar]
- Goldwater DS, Pavlides C, Hunter RG, Bloss EB, Hof PR, McEwen BS, Morrison JH. Structural and functional alterations to rat medial prefrontal cortex following chronic restraint stress and recovery. Neuroscience. 2009;164:798–808. doi: 10.1016/j.neuroscience.2009.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groc L, Choquet D, Chaouloff F. The stress hormone corticosterone conditions AMPAR surface trafficking and synaptic potentiation. Nat Neurosci. 2008;11:868–870. doi: 10.1038/nn.2150. [DOI] [PubMed] [Google Scholar]
- Gu J, Lee CW, Fan Y, Komlos D, Tang X, Sun C, Yu K, Hartzell HC, Chen G, Bamburg JR, Zheng JQ. ADF/cofilin-mediated actin dynamics regulate AMPA receptor trafficking during synaptic plasticity. Nat Neurosci. 2010;13:1208–1215. doi: 10.1038/nn.2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KM, Stevens JK. Dendritic spines of CA 1 pyramidal cells in the rat hippocampus: serial electron microscopy with reference to their biophysical characteristics. J Neurosci. 1989;9:2982–2997. doi: 10.1523/JNEUROSCI.09-08-02982.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichetovkin I, Grant W, Condeelis J. Cofilin produces newly polymerized actin filaments that are preferred for dendritic nucleation by the Arp2/3 complex. Curr Biol. 2002;12:79–84. doi: 10.1016/s0960-9822(01)00629-7. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P, Latimer LG. Neurochemical and behavioral effects of corticotropin-releasing factor in the ventral tegmental area of the rat. J Pharmacol Exp Ther. 1987;242:757–763. [PubMed] [Google Scholar]
- Kalivas PW, Lalumiere RT, Knackstedt L, Shen H. Glutamate transmission in addiction. Neuropharmacology. 2009;56(Suppl 1):169–173. doi: 10.1016/j.neuropharm.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Brain Res Rev. 1991;16:223–244. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- Kasai H, Matsuzaki M, Noguchi J, Yasumatsu N, Nakahara H. Structure-stability-function relationships of dendritic spines. Trends Neurosci. 2003;26:360–368. doi: 10.1016/S0166-2236(03)00162-0. [DOI] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, Moussawi K, Lalumiere R, Schwendt M, Klugmann M, Kalivas PW. Extinction training after cocaine self-administration induces glutamatergic plasticity to inhibit cocaine seeking. J Neurosci. 2010;30:7984–7992. doi: 10.1523/JNEUROSCI.1244-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb B, Whishaw IQ. Brain plasticity and behavior. Annu Rev Psychol. 1998;49:43–64. doi: 10.1146/annurev.psych.49.1.43. [DOI] [PubMed] [Google Scholar]
- Korotkova TM, Brown RE, Sergeeva OA, Ponomarenko AA, Haas HL. Effects of arousal- and feeding-related neuropeptides on dopaminergic and GABAergic neurons in the ventral tegmental area of the rat. Eur J Neurosci. 2006;23:2677–2685. doi: 10.1111/j.1460-9568.2006.04792.x. [DOI] [PubMed] [Google Scholar]
- Kourrich S, Rothwell PE, Klug JR, Thomas MJ. Cocaine experience controls bidirectional synaptic plasticity in the nucleus accumbens. J Neurosci. 2007;27:7921–7928. doi: 10.1523/JNEUROSCI.1859-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause M, Dent EW, Bear JE, Loureiro JJ, Gertler FB. Ena/VASP proteins: regulators of the actin cytoskeleton and cell migration. Annu Rev Cell Dev Biol. 2003;19:541–564. doi: 10.1146/annurev.cellbio.19.050103.103356. [DOI] [PubMed] [Google Scholar]
- Liston C, Gan WB. Glucocorticoids are critical regulators of dendritic spine development and plasticity in vivo. Proc Natl Acad Sci U S A. 2011;108:16074–16079. doi: 10.1073/pnas.1110444108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Shepard JD, Hall FS, Shaham Y. Effect of environmental stressors on opiate and psychostimulant reinforcement, reinstatement and discrimination in rats: a review. Neurosci Biobehav Rev. 2003;27:457–491. doi: 10.1016/s0149-7634(03)00073-3. [DOI] [PubMed] [Google Scholar]
- Lua BL, Low BC. Cortactin phosphorylation as a switch for actin cytoskeletal network and cell dynamics control. FEBS Lett. 2005;579:577–585. doi: 10.1016/j.febslet.2004.12.055. [DOI] [PubMed] [Google Scholar]
- Marinelli M, Piazza PV. Interaction between glucocorticoid hormones, stress and psychostimulant drugs. Eur J Neurosci. 2002;16:387–394. doi: 10.1046/j.1460-9568.2002.02089.x. [DOI] [PubMed] [Google Scholar]
- Martin S, Henley JM, Holman D, Zhou M, Wiegert O, van Spronsen M, Joels M, Hoogenraad CC, Krugers HJ. Corticosterone alters AMPAR mobility and facilitates bidirectional synaptic plasticity. PLoS One. 2009;4:e4714. doi: 10.1371/journal.pone.0004714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki M, Ellis-Davies GC, Nemoto T, Miyashita Y, Iino M, Kasai H. Dendritic spine geometry is critical for AMPA receptor expression in hippocampal CA1 pyramidal neurons. Nat Neurosci. 2001;4:1086–1092. doi: 10.1038/nn736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai H. Structural basis of long-term potentiation in single dendritic spines. Nature. 2004;429:761–766. doi: 10.1038/nature02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matus A. Growth of dendritic spines: a continuing story. Curr Opin Neurobiol. 2005;15:67–72. doi: 10.1016/j.conb.2005.01.015. [DOI] [PubMed] [Google Scholar]
- May RC. The Arp2/3 complex: a central regulator of the actin cytoskeleton. Cell Mol Life Sci. 2001;58:1607–1626. doi: 10.1007/PL00000800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee AW, Bredt DS. Assembly and plasticity of the glutamatergic postsynaptic specialization. Curr Opin Neurobiol. 2003;13:111–118. doi: 10.1016/s0959-4388(03)00008-4. [DOI] [PubMed] [Google Scholar]
- Meng Y, Zhang Y, Tregoubov V, Falls DL, Jia Z. Regulation of spine morphology and synaptic function by LIMK and the actin cytoskeleton. Rev Neurosci. 2003;14:233–240. doi: 10.1515/revneuro.2003.14.3.233. [DOI] [PubMed] [Google Scholar]
- Morales-Medina JC, Sanchez F, Flores G, Dumont Y, Quirion R. Morphological reorganization after repeated corticosterone administration in the hippocampus, nucleus accumbens and amygdala in the rat. J Chem Neuroanat. 2009;38:266–272. doi: 10.1016/j.jchemneu.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Morton WM, Ayscough KR, McLaughlin PJ. Latrunculin alters the actin-monomer subunit interface to prevent polymerization. Nat Cell Biol. 2000;2:376–378. doi: 10.1038/35014075. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Cheng Y, Ramm E, Sheng M, Walz T. Structure and different conformational states of native AMPA receptor complexes. Nature. 2005;433:545–549. doi: 10.1038/nature03328. [DOI] [PubMed] [Google Scholar]
- Ono S. Regulation of actin filament dynamics by actin depolymerizing factor/cofilin and actin-interacting protein 1: new blades for twisted filaments. Biochemistry. 2003;42:13363–13370. doi: 10.1021/bi034600x. [DOI] [PubMed] [Google Scholar]
- Pacchioni AM, Cador M, Bregonzio C, Cancela LM. A glutamate-dopamine interaction in the persistent enhanced response to amphetamine in nucleus accumbens core but not shell following a single restraint stress. Neuropsychopharmacology. 2007;32:682–692. doi: 10.1038/sj.npp.1301080. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Oxford,UK: 2009. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Bell K, Duffy P, Kalivas PW. Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. J Neurosci. 1996;16:1550–1560. doi: 10.1523/JNEUROSCI.16-04-01550.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard TD. Regulation of actin filament assembly by Arp2/3 complex and formins. Annu Rev Biophys Biomol Struct. 2007;36:451–477. doi: 10.1146/annurev.biophys.35.040405.101936. [DOI] [PubMed] [Google Scholar]
- Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–465. doi: 10.1016/s0092-8674(03)00120-x. [DOI] [PubMed] [Google Scholar]
- Pontrello CG, Ethell IM. Accelerators, Brakes, and Gears of Actin Dynamics in Dendritic Spines. Open Neurosci J. 2009;3:67–86. doi: 10.2174/1874082000903020067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad BM, Ulibarri C, Sorg BA. Stress-induced cross-sensitization to cocaine: effect of adrenalectomy and corticosterone after short- and long-term withdrawal. Psychopharmacology (Berl) 1998;136:24–33. doi: 10.1007/s002130050535. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Rodriguez A, Ehlenberger DB, Dammann M, McEwen BS, Morrison JH, Wearne SL, Hof PR. Repeated stress alters dendritic spine morphology in the rat medial prefrontal cortex. J Comp Neurol. 2008;507:1141–1150. doi: 10.1002/cne.21588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Persistent structural modifications in nucleus accumbens and prefrontal cortex neurons produced by previous experience with amphetamine. J Neurosci. 1997;17:8491–8497. doi: 10.1523/JNEUROSCI.17-21-08491.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Alterations in the morphology of dendrites and dendritic spines in the nucleus accumbens and prefrontal cortex following repeated treatment with amphetamine or cocaine. Eur J Neurosci. 1999;11:1598–1604. doi: 10.1046/j.1460-9568.1999.00576.x. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47(Suppl 1):33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Rouge-Pont F, Deroche V, Le Moal M, Piazza PV. Individual differences in stress-induced dopamine release in the nucleus accumbens are influenced by corticosterone. Eur J Neurosci. 1998;10:3903–3907. doi: 10.1046/j.1460-9568.1998.00438.x. [DOI] [PubMed] [Google Scholar]
- Rouge-Pont F, Marinelli M, Le Moal M, Simon H, Piazza PV. Stress-induced sensitization and glucocorticoids. II. Sensitization of the increase in extracellular dopamine induced by cocaine depends on stress-induced corticosterone secretion. J Neurosci. 1995;15:7189–7195. doi: 10.1523/JNEUROSCI.15-11-07189.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37:577–582. doi: 10.1016/s0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- Santos SD, Carvalho AL, Caldeira MV, Duarte CB. Regulation of AMPA receptors and synaptic plasticity. Neuroscience. 2009;158:105–125. doi: 10.1016/j.neuroscience.2008.02.037. [DOI] [PubMed] [Google Scholar]
- Schnell E, Sizemore M, Karimzadegan S, Chen L, Bredt DS, Nicoll RA. Direct interactions between PSD-95 and stargazin control synaptic AMPA receptor number. Proc Natl Acad Sci U S A. 2002;99:13902–13907. doi: 10.1073/pnas.172511199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann J, Yaka R. Prolonged withdrawal from repeated noncontingent cocaine exposure increases NMDA receptor expression and ERK activity in the nucleus accumbens. J Neurosci. 2009;29:6955–6963. doi: 10.1523/JNEUROSCI.1329-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seib LM, Wellman CL. Daily injections alter spine density in rat medial prefrontal cortex. Neurosci Lett. 2003;337:29–32. doi: 10.1016/s0304-3940(02)01287-9. [DOI] [PubMed] [Google Scholar]
- Shen HW, Toda S, Moussawi K, Bouknight A, Zahm DS, Kalivas PW. Altered dendritic spine plasticity in cocaine-withdrawn rats. J Neurosci. 2009;29:2876–2884. doi: 10.1523/JNEUROSCI.5638-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorg BA, Kalivas PW. Effects of cocaine and footshock stress on extracellular dopamine levels in the ventral striatum. Brain Res. 1991;559:29–36. doi: 10.1016/0006-8993(91)90283-2. [DOI] [PubMed] [Google Scholar]
- Swanson CJ, Baker DA, Carson D, Worley PF, Kalivas PW. Repeated cocaine administration attenuates group I metabotropic glutamate receptor-mediated glutamate release and behavioral activation: a potential role for Homer. J Neurosci. 2001;21:9043–9052. doi: 10.1523/JNEUROSCI.21-22-09043.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinny JD, Valentino RJ. Corticotropin-releasing factor promotes growth of brain norepinephrine neuronal processes through Rho GTPase regulators of the actin cytoskeleton in rat. Eur J Neurosci. 2006;24:2481–2490. doi: 10.1111/j.1460-9568.2006.05129.x. [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, Ary AW, Lominac KD. Homers regulate drug-induced neuroplasticity: implications for addiction. Biochem Pharmacol. 2008;75:112–133. doi: 10.1016/j.bcp.2007.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szumlinski KK, Dehoff MH, Kang SH, Frys KA, Lominac KD, Klugmann M, Rohrer J, Griffin W, 3rd, Toda S, Champtiaux NP, Berry T, Tu JC, Shealy SE, During MJ, Middaugh LD, Worley PF, Kalivas PW. Homer proteins regulate sensitivity to cocaine. Neuron. 2004;43:401–413. doi: 10.1016/j.neuron.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Tagliaferro P, Morales M. Synapses between corticotropin-releasing factor-containing axon terminals and dopaminergic neurons in the ventral tegmental area are predominantly glutamatergic. J Comp Neurol. 2008;506:616–626. doi: 10.1002/cne.21576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda S, Shen H, Kalivas PW. Inhibition of actin polymerization prevents cocaine-induced changes in spine morphology in the nucleus accumbens. Neurotox Res. 2010;18:410–415. doi: 10.1007/s12640-010-9193-z. [DOI] [PubMed] [Google Scholar]
- Toda S, Shen HW, Peters J, Cagle S, Kalivas PW. Cocaine increases actin cycling: effects in the reinstatement model of drug seeking. J Neurosci. 2006;26:1579–1587. doi: 10.1523/JNEUROSCI.4132-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA, Singh V, Crowder TL, Yaka R, Ron D, Bonci A. Corticotropin-releasing factor requires CRF binding protein to potentiate NMDA receptors via CRF receptor 2 in dopamine neurons. Neuron. 2003;39:401–407. doi: 10.1016/s0896-6273(03)00461-6. [DOI] [PubMed] [Google Scholar]
- Usui S, Konno D, Hori K, Maruoka H, Okabe S, Fujikado T, Tano Y, Sobue K. Synaptic targeting of PSD-Zip45 (Homer 1c) and its involvement in the synaptic accumulation of F-actin. J Biol Chem. 2003;278:10619–10628. doi: 10.1074/jbc.M210802200. [DOI] [PubMed] [Google Scholar]
- Van Troys M, Huyck L, Leyman S, Dhaese S, Vandekerkhove J, Ampe C. Ins and outs of ADF/cofilin activity and regulation. Eur J Cell Biol. 2008;87:649–667. doi: 10.1016/j.ejcb.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Wanat MJ, Hopf FW, Stuber GD, Phillips PE, Bonci A. Corticotropin-releasing factor increases mouse ventral tegmental area dopamine neuron firing through a protein kinase C-dependent enhancement of Ih. J Physiol. 2008;586:2157–2170. doi: 10.1113/jphysiol.2007.150078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Shaham Y, Zitzman D, Azari S, Wise RA, You ZB. Cocaine experience establishes control of midbrain glutamate and dopamine by corticotropin-releasing factor: a role in stress-induced relapse to drug seeking. J Neurosci. 2005;25:5389–5396. doi: 10.1523/JNEUROSCI.0955-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, You ZB, Rice KC, Wise RA. Stress-induced relapse to cocaine seeking: roles for the CRF(2) receptor and CRF-binding protein in the ventral tegmental area of the rat. Psychopharmacology (Berl) 2007;193:283–294. doi: 10.1007/s00213-007-0782-3. [DOI] [PubMed] [Google Scholar]
- Yao WD, Gainetdinov RR, Arbuckle MI, Sotnikova TD, Cyr M, Beaulieu JM, Torres GE, Grant SG, Caron MG. Identification of PSD-95 as a regulator of dopamine-mediated synaptic and behavioral plasticity. Neuron. 2004;41:625–638. doi: 10.1016/s0896-6273(04)00048-0. [DOI] [PubMed] [Google Scholar]