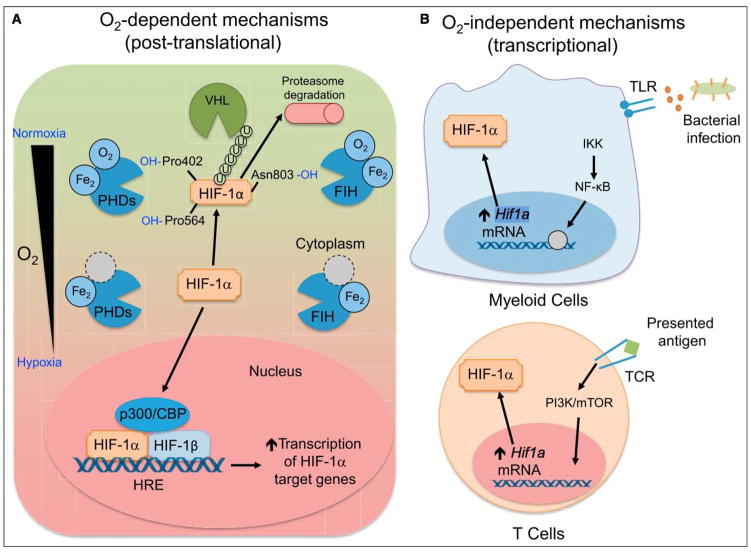
Figure 1. Mechanisms of HIF Stabilization by Immune Cells.
(A) O2 dependent. When oxygen is available, HIF-1α is hydroxylated by PHD, enzymes that depend on oxygen and iron as cofactors. When prolylhydroxylated, HIF-1a is polyubiquitinated by VHL, marking it for proteasomal degradation. FIH hydroxylates HIF-1α at asparagine 803, which does not lead to polyubiquitination, but instead blocks interactions between HIF-1α and p300/CBP, a member of the HIF complex that acts as a coactivator of target gene transcription. When oxygen tension drops, PHD and FIH activity is inhibited, leading to HIF-1α accumulation and nuclear translocation, heterodimerization with HIF-1β, and recruitment of p300/CBP. The HIF transcriptional complex binds to hypoxia-response elements (HREs) to control target gene expression. (B) O2 independent. Bacterial products are recognized by TLRs expressed on myeloid cells, signaling through NF-κB to increase Hif1a transcription. In a similar fashion, TCR ligation upon antigen presentation on T lymphocytes leads to increased Hif1a transcription and HIF-1a protein accumulation, even in the presence of oxygen. While the mechanism of Hif1a mRNA induction is not known, activation of PI3K and mTOR appear to be involved in TCR-mediated induction of HIF-1α.