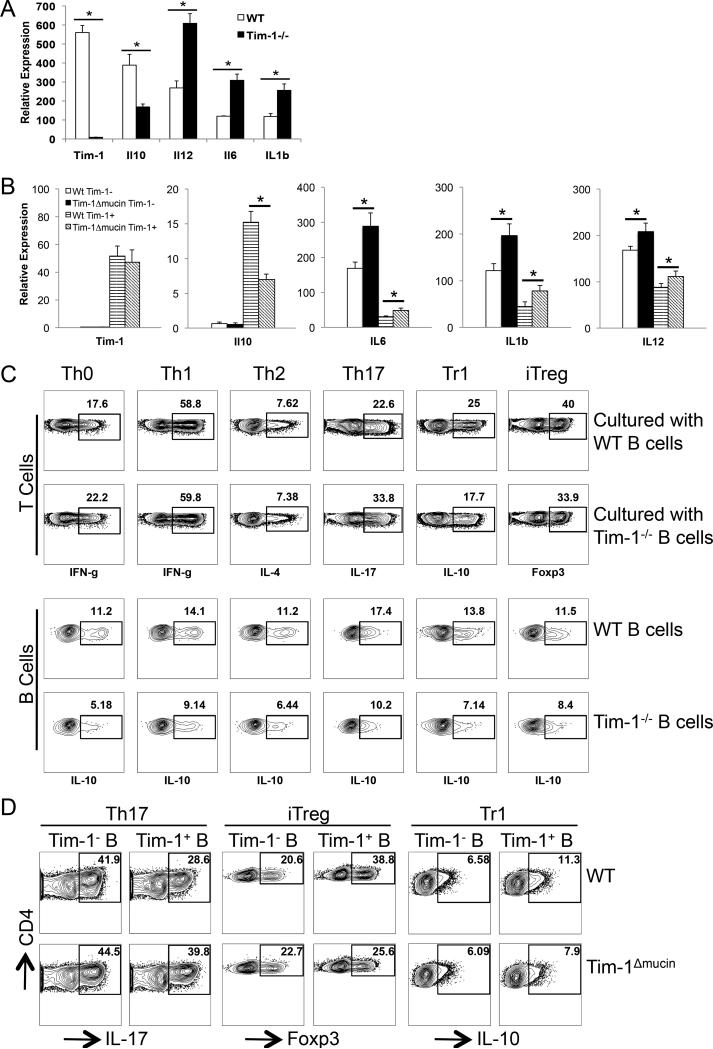
Figure 3. Tim-1 expression or defects affects the balance between regulatory and inflammatory cytokines in B cells that subsequently alter T cell responses.
A) Purified splenic CD19+ B cells from WT or Tim-1−/− mice were cultured in the presence of anti-IgM ((Fab’)2 fragment) for 24 h. Total RNA was isolated, and relative expression (mean ± SEM; n = 5) of Tim-1, IL10, IL12, IL6, and IL1b mRNA was measured by realtime PCR. * P < 0.01. B) WT total CD4+ T cells (10 x 106/mouse) were co-transferred together with WT or Tim-1Δmucin CD19+ B cells (20 x 106) into Rag1−/− mice. One day after, mice were immunized with MOG35-55/CFA to induce EAE. At the peak of disease, splenic Tim-1+ and Tim-1− CD19+ B cells were purified from WT and Tim-1Δmucin groups of mice. Total RNA was isolated, and relative expression (mean + SEM; n = 5) of Tim-1, IL10, IL12, IL6, and IL1b mRNA was measured by realtime PCR. * P < 0.01. C) WT naïve CD4+ T cells were cultured with splenic CD19+ B cells purified from WT or Tim-1−/− IL-10GFP/+ mice in the presence of anti-CD3 under Th0 (no cytokine), Th1 (IL-12 + anti-IL-4), Th2 (IL-4 + anti-IL-12/anti-IFN-γ), Th17 (TGF-β1 + IL-6), Tr1 (TGF-β1 + IL-27), and iTreg (TGF-β1) conditions. After culture for 4 days, production of indicated cytokines in T cells and IL-10 (GFP+) in B cells was measured by flow cytometry after intracellular cytokine staining. Representative of 5 independent experiments was shown. D) WT CD4+ naïve T cells were cultured with Tim-1+ or Tim-1− B cells purified from WT in the presence of anti-CD3 under Th17, Tr1, and iTreg conditions. After culture for 4 days, production of indicated cytokines in T cells was measured by flow cytometry after intracellular cytokine staining. Representative data from 3 independent experiments are shown.