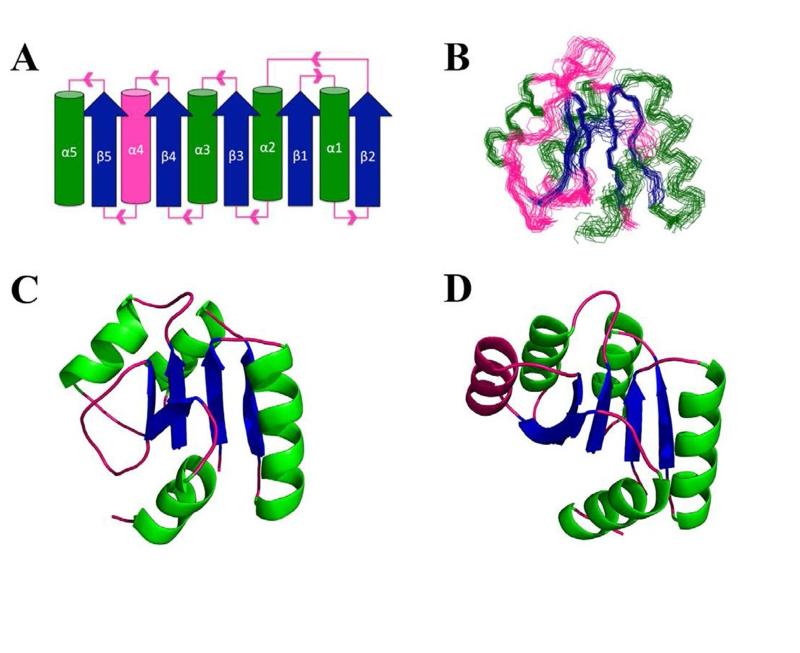
Figure 2.
Fold and NMR structure of Sma0114. (A) Topology diagram for the canonical α5/β5 Rossman fold of receiver domains. Parallel β-sheets (blue) are surrounded by α-helices (green) with pink segments indicating loops. The fourth α-helix which is disordered in Sma0114 is also indicated in pink. (B) Backbone representation of the 20 lowest energy NMR structures. Regular secondary structure elements 9-15 (β1), 19-31 (α1), 34-39 (β2), 42-51 (α2), 55-60 (β3), 70-78 (α3), 81-86 (β4), 102-105 (β5), 110-117 (α5) were used to superpose the structures. (C) NMR structure of Sma0114 closest to the ensemble average. The fourth α-helix is replaced by a disordered segment. (D) NMR structure of Spo0F (PDB accession code 1NAT) (47). The fourth α-helix is shown in pink.