Abstract
Many mosses of the family Splachnaceae are entomophilous and rely on flies for spore dispersal. Splachnum ampullaceum produces a yellow- or pink-coloured hypophysis that releases volatile compounds, attracting flies to the mature moss. The biosynthetic sources of the visual and aromatic cues within the hypophysis have not been identified, and may be either symbiotic cyanobacteria or chromoplasts that break down lipids into volatile compounds. Here, we used transmission electron microscopy and gas chromatography-mass spectrometry (GC-MS) to investigate the sources of these attractants, focusing on different tissues and stages of maturation. Microscopy revealed an abundance of plastids within the hypophysis, while no symbiotic bacteria were observed. During plant maturation, plastids differentiated from amyloplasts with large starch granules to photosynthetic chloroplasts and finally to chromoplasts with lipid accumulations. We used GC-MS to identify over 50 volatile organic compounds from mature sporophytes including short-chain oxygenated compounds, unsaturated irregular terpenoids, fatty acid-derived 6- and 8-carbon alcohols and ketones, and the aromatic compounds acetophenone and p-cresol. The hypophysis showed localised production of pungent volatiles, mainly short-chain fermentation compounds and p-cresol. Some of these volatiles have been shown to be produced from lipid oxidase degradation of linolenic acid within chromoplasts. However, other compounds (such as cyclohexanecarboxylic acid esters) may have a microbial origin. Further investigation is necessary to identify the origin of fly attractants in these mosses.
Keywords: Development, dung moss, hypophysis, plastid, Splachnum, sporophyte, volatiles
INTRODUCTION
Approximately half of the species in the moss family Splachnaceae are entomophilous, having their spores dispersed by flies (Diptera; Koponen 1990; Marino 1991). Entomophilous species grow on organic substrates such as dung, old bones and owl pellets, and have mature sporophytes with an inflated, often brightly coloured swelling (hypophysis) just proximal to the spore-containing capsule. The sporophytes of all entomophilous species so far examined release volatile chemicals that attract flies (Pyysalo et al. 1978, 1983; Marino et al. 2009). Sporophyte odour in the Splachnaceae is novel among mosses, with both visual and olfactory cues appearing integral to fly attraction (Koponen 1990; Marino et al. 2009). The complex and diverse volatile compounds released by mature Splachnaceae hypophyses suggest chemical mimicry of herbivore dung among several genera (e.g. Splachnum and Tayloria) and of carrion among species in the genus Tetraplodon (Pyysalo et al. 1978, 1983; Marino et al. 2009). The sources of these chemical cues and the enzymatic pathways that lead to their production are unknown.
Characteristic fly-attracting odour and colour cues appear only in mature hypophyses (i.e. when spores are ready to be dispersed), and correspondingly, we might expect that the ultrastructure of hypophysis cells varies throughout sporophyte development, with features appearing near or at maturity possibly being related to the production of secondary metabolites. For example, plastids can become differentiated and undifferentiated during plant development and as a response to environmental change (Hayashi & Nishimura 2012). In particular, chromoplasts are known to impart colour to plants and produce volatile compounds (Bathgate et al. 1985; Barsan et al. 2010; Bian et al. 2011); therefore, they may be more abundant within the hypophysis of mature sporophytes. Histological studies have associated starch deposits within glandular floral tissues (osmophores) with the emission of volatile compounds, with dramatic reduction of starch occurring during the course of floral maturation and scent emission (Vogel 1990). Thus, by analogy to the ontogeny of floral scent, the consumption of plastidic starch granules could contribute to the biosynthesis and emission of volatiles in the mature hypophyses of Splachnum ampullaceum. Alternatively, symbiotic bacteria (e.g. cyanobacteria) might be the source of odours – and possibly also characteristic colours – in mature hypophyses.
Here, we examine ultrastructural changes in S. ampullaceum hypophyses at different maturation stages, focusing on characters that appear during the mature stage, when characteristic odours and colours are produced. In addition, we analyse the volatiles released by different tissues of mature S. ampullaceum sporophytes to better localise the sources of their production.
MATERIAL AND METHODS
Sample collection
A population of S. ampullaceum growing on the summer faeces of a moose (Alces alces) was collected from near Salmonier Nature Park (47°15.03′7N, 53°18.424′W), NL, Canada, in June 2011 and maintained in the lab. The population of S. ampullaceum was at the very earliest stages of sporophyte development when collected. Sporophytes were sampled from this colony at eight different stages of maturation, identified as stages 1 to 8 (Table 1).
Table 1.
Maturation stages of Splachnum ampullaceum sporophytes.
| maturation stage | collection date | sporophyte length (cm) | number of specimens | sporophyte appearance |
|---|---|---|---|---|
| Stage 1 | 17-06-2011 | 1.3–1.9 | 5 | hypophysis not swollen |
| Stage 2 | 17-06-2011 | 2.4–4.0 | 5 | hypophysis not swollen |
| Stage 3 | 17-06-2011 | 2.5–3.5 | 5 | hypophysis slightly swollen |
| Stage 4 | 05-07-2011 | 2.5–3.4 | 3 | hypophysis with medium swelling |
| Stage 5 | 05-07-2011 | 2.9–3.0 | 3 | hypophysis with medium swelling, operculum beginning to form |
| Stage 6 | 05-07-2011 | 2.9–3.5 | 3 | hypophysis fully swollen, operculum fully developed |
| Stage 7 | 05-07-2011 | 2.5–3.7 | 3 | hypophysis fully swollen, no operculum |
| Stage 8 | 21-07-2011 | 3.9–4.7 | 4 | red, fully mature, hypophysis fully swollen, no operculum |
Sample fixation and embedding
All sporophytes were dissected into three regions: the hypophysis (distal end, including the capsule, when present), the base of the hypophysis (proximal end, below the sporangium) and the seta. The hypophysis was considered to be the area near the distal end of the sporophyte in immature samples, or the area exhibiting swelling in more mature individuals. Sporophyte fragments were fixed in 2.5% glutaraldehyde in 1 M sodium cacodylate buffer for at least 24 h at 4 °C. Tissues were then immersed in 1 M sodium cacodylate buffer for 24 h prior to post-fixation in 1% OsO4 in the same buffer, for 2 h at room temperature. After rinsing with distilled water, samples were dehydrated in an ascending gradient of ethanol (minimum of 6 h per wash), and taken through three changes of Epon resin (1 h each) before final embedding.
Light and transmission electron microscopy
Resin blocks were trimmed and sectioned on a LKG Bromma 8800 ultramicrotome (Bromma, Stockholm, Sweden). Semi-thin (1 μm) sections were stained with 1% toluidine blue in 1% sodium borate for observation and imaging using a Zeiss Axioscope A1 (Zeiss, Oberkochen, Germany). Serial semi-thin sections were made through segments of each block, and the number of plastids visible along the periphery of lumina (i.e. within one or more cells bordering individual luminae in transverse sections) in the epidermis, cortex and central strand were quantified.
For transmission electron microscopy (TEM), 70-nm thick sections from various locations along the length of setae and hypophyses were placed on copper grids and stained with 3% uranyl acetate in 30% ethanol for 5 min. After rinsing, a second 5 min staining with 38% lead citrate buffered with sodium hydroxide was conducted. Sections were observed and imaged using a Philips 300 TEM (Philips, Amsterdam, the Netherlands).
Analysis of volatile compounds
A total of 40 individuals of S. ampullaceum with mature sporophytes were separated manually from a large population collected in August 2013 near Salmonier Nature Park (47°15.037′N, 53°18.424′W), NL, Canada. These plants were dissected with a sterile razor blade into (1) gametophytic tissues with attached rhizoids, (2) setae, (3) hypophyses + capsules and (4) capsules alone. Dissected tissues were placed into clean, oven-baked (50 °C) 1.5 ml glass autosampler vials, sealed with small gaskets cut from nylon resin oven bags (Reynolds, Inc.), and left to equilibrate under a 40 W light at 30 °C for 30 min. In addition, 30 uncut, mature sporophytes were placed into a clean, oven-baked 8.5 ml vial, to be used as an intact positive control sample. We exposed solid phase microextraction (SPME) fibres (65 μ polydimethylsiloxane/divinylbenzene fibre; Supelco, Inc., Oakville, Ontario, Canada) within the equilibrated headspace of each sample for an additional 30 min. Ambient control samples were taken from empty glass vials of both sizes as described above.
Trapped volatiles were analysed using a GC-17a gas chromatograph (GC) coupled with a QP5000 quadrupole, electron ionisation mass spectrometer (MS) as a detector (Shimadzu Scientific Instruments, Columbia, MD, USA), scanning from m/z 40–350 at 70 electron volts. Volatiles were desorbed from the solid phase microextraction (SPME) fibre by injecting (splitless) into a hot (240 °C) injection port, then separated on a polar GC column (ECWax – ethylene glycol as stationary phase; W.R. Grace and Associates, Columbia, MD, USA) with helium (99.999% purity) as mobile phase at a constant flow of 1 ml min−1 with a split ratio of 12:1. The GC oven temperature programme was held at 40 °C for 3 min, followed by an increase of 10 °C min−1, ending with a 5 min hold at 260 °C. Duplicate samples from intact sporophytes were run at slower (5 °C min−1) temperature ramps to ensure that additional GC peaks had not been obscured by co-eluting peaks. Actual moss volatiles not present in ambient controls were identified by comparing absolute retention index and mass spectra with those of authentic standards whenever possible, as well as with NIST and Wiley electronic mass spectra libraries. One such compound, 6-methyl-5-hepten-2-one, is a common contaminant, and was scored only if present at three-fold larger amounts than in the ambient control. Compounds that could not be identified in this way are presented as mass spectral data with retention times converted to standard Kovats indices using a ladder of increasing alkane standards (see Majetic et al. 2010). Total ion chromatogram peak areas were manually integrated using GCMSolutions software (Shimadzu Scientific Instruments).
Additional analyses were performed using another population of S. ampullaceum collected in August 2002, 20 km from Fort Assiniboine, AB, Canada (54°18′N, 114°50′W). The sticky spore mass was extruded from 50 dissected capsules by applying faint pressure to the capsule walls with a sterilised watchmaker’s forceps under magnification. Two replicate samples consisting of 50 capsules and hypophyses each were dissected from the remaining sporophytic tissue (setae), and each were placed in a 4 × 4 cm nylon resin oven bag (Reynolds) closed with an impulse heat sealer. Dissected samples were analysed with SPME-GC-MS under conditions similar to those described above (e.g. starting oven temperature 60 °C instead of 40 °C, otherwise the same temperature programme), using a Hewlett-Packard 5890 Series II GC (Hewlett-Packard, Palo Alto, CA, USA) interfaced with a quadrupole, electron impact mass selective detector (HP 5972). Chromatographic data were analysed using HP Chemstation software.
RESULTS
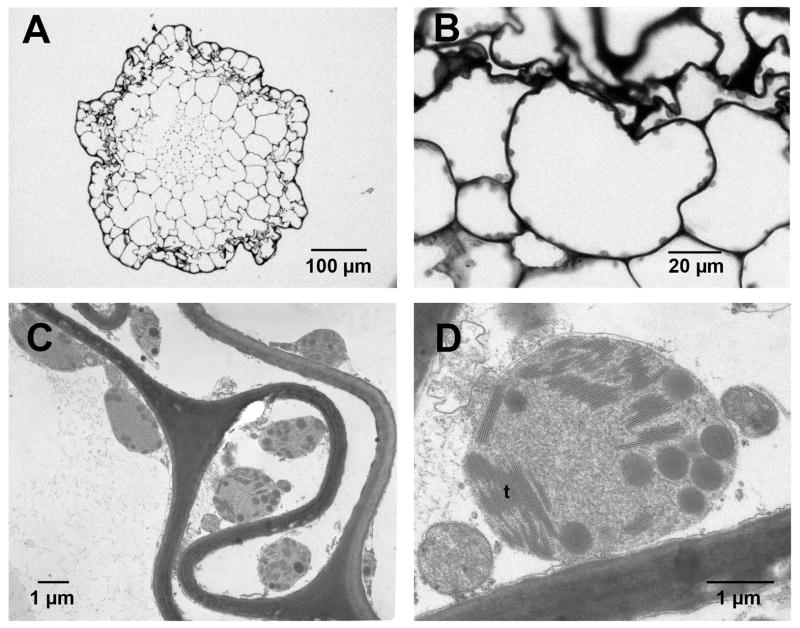
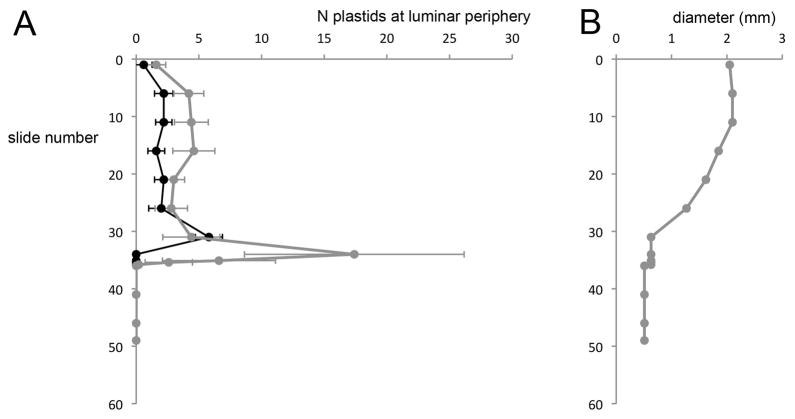
Transverse sections through mature hypophyses and setae revealed cells that appeared mostly senescent, and that lacked obvious nuclei and organelles, with the exception of abundant structures bearing thylakoid membranes (Fig. 1). Using fluorescence microscopy, we confirmed that these structures contained chlorophyll but lacked phycocyanin (data not shown); therefore, they were more likely to be plastids than cyanobacteria. Serial transverse sections through the length of the sporophyte indicated that plastids were more abundant in the hypophysis (especially at its proximal end) than in the setae, and especially in cells of the epidermis and cortex (Fig. 2). The presence of abundant plastids in mature sporophytes suggests their involvement in the production of chemical cues, as well as in the characteristic colouration of mature hypophyses.
Figure 1.
Light micrographs (LM) and transmission electron micrographs (TEM), transverse sections of hypophysis of mature S. ampullaceum sporophyte. (A) LM, proximal end of the hypophysis. (B) LM, cells in the outer cortex and epidermis of the hypophysis. Plastids are apparent along the periphery of lumina. (C) TEM, plastids near the moss cell wall. (D) Plastid, with apparent thalakoid membranes (t) plastoglobuli.
Figure 2.
Plastid abundance along the length of a segment of mature sporophyte, from the mid-hypophysis to the distal end of the seta, in a representative specimen of S. ampullaceum. Trends from two other specimens are similar. (A) The number of plastids (average ± SE of the mean, N = 5 counts per slide) visible along the periphery of luminar space in either the epidermis and cortex (grey) or central strand (black) in serially sectioned tissues, from the mid-hypophysis (slide number 0) to a few mm below the proximal end of the hypophysis (slide number 49). (B) Corresponding diameter of the sporophyte cross-section for each slide used in plastid quantifications, providing an indication of the distance along the sporophyte (i.e. from the enlarged midpoint of the hypophysis to the thin seta).
The TEM revealed structural changes in plastids during sporophyte development and maturation. We classified plastids into three types according to prominent characters, as described below. At some stages of development, more than one type of plastid could be observed; here, we group developmental stages according to the most common type represented therein. Our description focuses on the proximal end of the hypophysis, given that plastids were most abundant in this region. Plastids in the seta, when observed, were of the same type as in the hypophysis.
Cells in the distal-most third of the most immature sporophytes (stages 1 to 3) contained obvious cytoplasm and organelles, including plastids with large starch granules (Fig. 3A–D). Starch granules varied in size and number in the sectioned plastids observed, and occupied a variable amount of space in the organelle. As sporophytes matured, the size of starch granules diminished, and thylakoid membranes developed (Fig. 3C, D). Plastids undergoing division were also evident in immature sporophytes (Fig. 3B).
Figure 3.
Transmission electron micrographs, transverse sections of cells within young hypophyses (stages 1–3). Cells contain abundant cytoplasm (c). (A) Stage 1: Plastid (p) with both starch granules (s) and poorly developed membranes. (B) Stage 1: Plastids with numerous starch granules; some appear to be dividing (arrows). (C) Stage 2: Plastids containing starch granules and/or further developed thylakoid membranes (t). (D) Stage 3: Plastids with starch granules of various sizes, and densely packed membranes. w: cell wall.
In intermediate stages of sporophyte development (stages 4 to 7), cellular material was visible, although reduced, and most plastids contained abundant, well-developed thylakoid membranes (Fig. 4A–C). Small starch granules were present in some plastids at stage 4 (Fig. 4A), but were not seen in later stages.
Figure 4.
TEM, transverse sections of cells within the hypophyses of stage 4–8 individuals. Cytoplasm is reduced. (A) Stage 4: Plastid with small starch granule and abundant thylakoid membranes. (B) Stage 5: Plastid with thylakoid membranes. (C) Stage 7: Plastid with empty spaces between membranes as well as small, plastoglobuli. (D) Stage 8: Plastid with few membranes and large plastoglobuli.
In mature hypophyses (stage 8), plastids exhibited severe membrane degradation and showed evidence of lipid accumulation in the form of plastoglobuli (Fig. 4D). As hypophyses matured, cellular content regressed even further.
Volatile compound analysis
The SPME-GC-MS analyses revealed a total of 69 volatile organic compounds (VOC) from whole or dissected tissues of S. ampullaceum (Table S1). Gametophytes (green tissues plus rhizoid traces) emitted ten VOC, nearly all of which were monoterpene (C10H16) or sesquiterpene (C15H24) hydrocarbons. In contrast, mature sporophytes emitted 51 different VOC, only three of which overlapped with gametophyte chemistry, and did so with 14-fold larger total (equilibrated) peak areas when compared with gametophytic tissues from the same number of individuals (Table S1). Volatiles identified from sporophytes included short-chain oxygenated compounds (e.g. 2-methyl butanol), unsaturated irregular terpenoids (e.g. carotenoid-derived 6-methyl-5-hepten-2-one and its associated alcohols), fatty acid-derived 6- and 8-carbon alcohols and ketones, and the aromatic compounds acetophenone and p-cresol. The most unusual class of VOC emitted by sporophytes was a group of ten compounds with very distinctive mass spectra, suggesting esters of cyclohexanecarboxylic acid, which could not be verified due to the lack of commercially available authentic standards.
Among dissected tissues, total emissions from cut setae were comparable to those of gametophytes and were largely limited to terpenoids and C8 mushroom-scented alcohols and ketones (Table S1). Dissected hypophyses (with or without capsules attached) were eight-fold more strongly scented than setae, and were the exclusive source of acetophenone as well as the irregular terpenoids and putative cyclohexanecarboxylic acid esters that dominated emissions from intact sporophytes, whereas p-cresol was only present when the capsule was included. Interestingly, a unique set of generic carboxylic acids (butyric, pentanoic, hexanoic acid) were detected only when capsules were dissected from the hypophyses, and along with n-alkanes were emitted in large amounts, comparable to intact sporophytes (Table S1).
The second set of dissections performed on a different accession of S. ampullaceum and analysed on a different GC-MS system yielded very similar results, this time including volatiles emitted by spores (Table S2). A total of 57 VOC were found, including many of the characteristic compounds identified in Table S1, as well as 15 VOC identifiable as saturated long-chain hydrocarbons, due to their distinctive mass ion fragments (m/z 43, 57, 71, 85, etc.). Most of these hydrocarbons are attributable to the capsules and especially the spores, whereas the irregular terpenoids and short-chain fermentation-related compounds (isopropanol, 2-methyl butanol) again were associated only with the hypophyses (Fig. 5, Table S2).
Figure 5.
Chemical dissection of volatiles released by different parts of the sporophyte (insert). Total ion chromatograms are shown from (A) freely extruded spores, (B) dissected capsule and (C) the remaining hypophysis. The horizontal axis is time (and GC oven temperature, increasing from 60 °C to 240 °C at 10 °C min−1. The vertical axis is signal intensity (detector voltage) – note that the vertical scales are not the same for the strongly scented hypophysis and the more weakly scented spores. Pie charts indicate relative contributions of different compound classes to total emissions (see legend), and are scaled to total GC-MS peak areas – note that capsules and especially spores are dominated by saturated long-chain hydrocarbons.
DISCUSSION
The histological and ultrastructural examination of S. ampullaceum sporophytes has revealed the potential for a relationship between plastid distribution and the characteristic odour (and possibly also colour) production in this species. In mature S. ampullaceum sporophytes, the production of pungent volatile compounds likely to attract flies (short-chain fermentation compounds, p-cresol) is mainly localised to the swollen hypophysis, with little chemical release from the seta. Intriguingly, the hypophysis was also found to contain the highest number of plastids, which are responsible for the biosynthesis of carotenoid-derived volatiles (Walter et al. 2010) identified in our chemical analyses.
Different types of plastids, mainly characterised by their contents, can be found throughout plant tissues and life stages. Amyloplasts contain large starch granules and may function in plastid propagation and gravity sensing in addition to being major sites of starch storage for many plants (Morita & Tasaka 2004; Sagasaka 2008; Inaba & Ito-Inaba 2010). Chloroplasts contain thylakoid membranes in which the photosynthetic pigment chlorophyll is embedded; chlorophyll harnesses solar energy and transforms it into useful compounds (Inaba & Ito-Inaba 2010). Chromoplasts, commonly found in fruits, are pigment-containing, non-photosynthetic plastids that develop from chloroplasts as the fruit ripens (Bathgate et al. 1985; Bian et al. 2011). A number of structural changes occur as chloroplasts differentiate into chromoplasts. First, the internal membranes break down, resulting in the formation of lipid-containing granules, the plastoglobuli (Thompson et al. 1998). This is followed by an accumulation of carotenoid pigments, which imparts colour, commonly yellow and red derivatives, to plant tissues (Bathgate et al. 1985; Barsan et al. 2010; Bian et al. 2011). One such pigment, lycopene, is a known precursor of C8 alcohol and ketone aroma constituents of tomato and melon fruits (Lewinsohn et al. 2005; Tiemann et al. 2006). A family of enzymes, namely lipid oxygenases (LOX), has been associated with chromoplasts and wound-induced volatile production in green plant tissues. These enzymes break down lipids, creating volatile compounds associated with plant and fruit odours (Thompson 1998; Zhang et al. 2009; Barsan et al. 2010; Bian et al. 2011). In the present study, different plastid types were observed throughout sporophyte development, as described below.
Amyloplasts in immature sporophytes
The observation of plastids in immature S. ampullaceum sporophytes is consistent with a report of plastid presence throughout early sporophyte development in mosses outside of the family Splachnaceae, including Funaria flavicans, Bartramia pomiformis, Pogonatum pensilvanicum, Dicranum scoparium, Andreaea sp. and Sphagnum palustre (Bold 1940). The most prevalent plastids seen in immature S. ampullaceum sporophytes are likely amyloplasts, which are characterised by the presence of large starch granules (Hayashi & Nishimura 2009). Amyloplasts contain few thylakoid membranes and have a reduced capacity for photosynthesis; rather, they are thought to function mainly in gravitropism and plastid biogenesis (Morita & Tasaka 2004; Sagasaka 2008; Inaba & Ito-Inaba 2010). The starch-statolith theory suggests that starch causes amyloplasts to sediment, triggering a gravitropic response in shoot endodermal cells (Morita & Tasaka 2004; Morita 2010). Amyloplast sedimentation occurs in restricted areas where growth direction is important, such as root and shoot tips (Sack et al. 2001; Morita 2010). The areas sectioned in the young sporophytes were much closer to the shoot tip than those sectioned in more mature hypophyses, so it is possible that these amyloplasts were involved in gravitropism. Amyloplasts in young plant tissues may also proliferate and then differentiate into chloroplasts or other plastids as the tissue matures (Sagisaka 2008). Accordingly, we observed both amyloplasts undergoing division, and a progression from amyloplasts with large starch granules and few or no thylakoid membranes to plastids with small granules and more abundant thylakoid membranes as sporophytes matured.
Chloroplasts in hypophyses undergoing swelling and spore formation
Splachnum ampullaceum individuals in the process of hypophysis swelling and spore formation contained chloroplasts with well-developed thylakoid membranes. Chloroplasts at this stage are likely the product of amyloplast differentiation, as evidenced by the intermediate characters observed in plastids from stage 4 individuals (i.e. small starch granules and abundant thylakoid membranes). Chloroplasts appear to have an elongated shape that, although not as pronounced as in the leaves of plants such as birch (Dodge 1969), could provide a larger membrane surface area and allow for more efficient photosynthesis. Although part of the nutritional demands of the sporophyte is met through transfer from the gametophyte (Renault et al. 1992), chloroplasts may also contribute to the nutritional requirements of spore formation at this stage. Similar observations were made in the swelling hypophysis of F. flavicans: all cells in this region were deemed to be actively photosynthetic, based on fixed and stained sections (Bold 1940).
Plastids in fully swollen and mature hypophyses
In mature S. ampullaceum hypophyses, cellular content was much reduced, although abundant plastids remained, particularly in the periphery of the hypophysis; a similar observation was made in the hypophysis of F. flavicans (Bold 1940). The cells in mature S. ampullaceum hypophyses resembled those of yellowing birch leaves, where the cytoplasm retreats to the edges of cell walls (Dodge 1969). The tissues of mature sporophytes appear to be undergoing senescence, which is to be expected as the capsule dehisces at this stage (Koponen 1990).
Chloroplasts in mature S. ampullaceum sporophytes follow a degradation pattern that has been observed in yellowing birch leaves, where the thylakoid membranes first break down, followed by the rest of the organelle (Dodge 1969). In senescing leaves, the breakdown of chloroplast membranes is thought to result in lipid accumulation within granules (Thompson et al. 1998). Further, the loss of chlorophyll contained within thylakoid membranes leads to the loss of green colour in these leaves (Bathgate et al. 1985). The nutritional requirements of maturing sporophyte hypophyses probably diminish, and consequently, efficient photosynthesis may not be important in these tissues.
The combined decrease in thylakoid membranes and appearance of plastoglobuli observed in mature sporophyte chloroplasts is characteristic of the early stages of chromoplast differentiation from chloroplasts (Bathgate et al. 1985; Bian et al. 2011). Chromoplasts are non-photosynthetic plastids that retain carotenoids, often giving tissues a yellow, orange or red colour (Barsan et al. 2010). The mature S. ampullaceum hypophysis appears yellow to sometimes pink, and this colour is important in attracting the flies involved in spore dispersal (Troilo & Cameron 1981). Chromoplasts have also been linked with odour production in the fruits of many plants (Zhang et al. 2009; Barsan et al. 2010). The membrane breakdown and lipid accumulation provides free fatty acids for enzymes, such as those in the LOX family, to use as substrates in the production of volatile organic compounds (Thompson et al. 1998; Zhang et al. 2009).
Functional and biosynthetic aspects of S. ampullaceum volatiles
Fly attraction to the sporophytes of S. ampullaceum is mediated by their pungent scent as well as their yellow-green colour (Marino et al. 2009). Considering the complex blend emitted by these sporophytes (nearly 70 VOC; Table S1), there are many candidates for specific fly attractants, including p-cresol, a common volatile constituent of mammal urine, faeces and flowering plants that mimic these substrates to attract fly pollinators (Jürgens et al. 2006). Trimethyl amine, the compound we expected to find due to the ‘dead fish’ quality of the scent of S. ampullaceum, was not identified in our analyses. However, 3,7-dimethyl-2-octene, a putatively identified VOC that comprised 30% of total sporophyte and hypophysis emissions, may be responsible for this fishy note, as a similar compound (2,6-dimethyl-3-octene) has been identified from fish oil (Hartvisgen et al. 2000). These VOC and several volatile unknowns with similar mass spectra (Tables S1, S2) show structural similarity to the branched apocarotenoids, 6-methyl-5-hepten-2-one and 6-methyl-5-hepten-2-ol identified from S. ampullaceum hypophyses. Our dissections revealed that highly pungent organic acids (butyric, pentanoic and hexanoic acids; see Table S1) identified in previous analyses of Splachnaceae volatiles by Pyysalo et al. (1978, 1983) are only released when hypophyses and capsules are wounded, as they would have been in solvent extractions performed by those authors. Another potential source of pungency in unwounded sporophytes would be the strongly mushroom-scented compounds octan-3-one and 1-octen-3-ol, which are biosynthesised from linolenic acid through the action of LOX, and have several ecological roles, including signalling between plants and fungi in the rhizosphere (Kishimoto et al. 2007). Lipid metabolism was shown to be highly active in chromoplasts (Bian et al. 2011), and is likely to be important in the production of volatiles in entomophilous mosses. However, the long-chain saturated hydrocarbons that dominated the dissected capsule and spore samples are neither pungent in scent (as they have very low volatility) nor are they unusual to the Splachnaceae. These generic compounds are common to the spores of non-entomophilious mosses (e.g. Polytrichum commune) as well as ferns and liverworts (reviewed in Karunen 1974). The spore mass of S. ampullaceum and other entomophilous Splachnaceae is extruded as a sticky mass, and the surprisingly large alkane peaks in the GC-MS analyses of spore headspace (Fig. 5) may indicate abundant hydrocarbon deposits on the spore surface.
Additionally, symbiotic microbes associated with the mosses might be involved in the production of VOC. Para-cresol is known to result from anaerobic microbial degradation of tyrosine (see Whittle et al. 2000). Unlike the compounds listed above, we did not find a suite of volatile aromatic metabolites related to cresol, other than acetophenone (Table S1), suggesting the absence of an active pathway. We did find several unknown compounds with mass spectra similar to that of beta-cyclocitral, an apocarotenoid known to be produced by cyanobacteria (Tellez et al. 2001; Jüttner et al. 2010). Finally, to our knowledge, cyclohexanecarboxylic acid esters have not previously been identified in mosses or flowers, and might reasonably be supposed to have a microbial origin. Cyclohexanecarboxylic acid has been identified (along with other acids) in the urine of red deer (elk), where it or its bacterially modified derivatives may function as a sexual or social signal (Bakke & Ficenschou 1990). If the flies that disperse the spores of S. ampullaceum are attracted by moose urine as well as faeces, these compounds would constitute strong candidates for agents of chemical mimicry.
Our analyses have raised additional questions concerning the ontogenic sources of VOC in the sporophytes of S. ampullaceum. Further investigation is needed to explore the potential involvement of chromoplasts and plant-associated microbes in the production of fly-attracting volatiles in this and other species of dung mosses. Genomic, proteomic and transcriptomic studies may provide useful insights into the biochemical processes that give rise to the unique odours produced by entomophilous mosses.
Supplementary Material
SPME-GC-MS analyses and identity of 69 VOC from whole or dissected tissues of S. ampullaceum.
Summary of GC-MS data for dissected sporophytes and spores.
Acknowledgments
We thank P. J. Coates and L. Chatman for specimen preparation and histology. K. Williams and S. Tucker assisted with transmission electron microscopy at the EM Unit, Memorial University of Newfoundland. D. Campbell and J. Ehrman performed fluorescence microscopy at Mount Allison University. Financial support was provided through a Natural Sciences and Engineering Research Council of Canada Discovery Grant to P. Marino, and by two South Carolina BRIN/EPSCoR CRP grants to R. Raguso and P. Marino (US National Science Foundation (NSF) EPSCoR Grant No. EPS-0132573 and US National Institute of Health (NIH) Grant RR-P20 RR 016461, in association with the BRIN Program of the National Center for Research Resources).
References
- Bakke JM, Ficenschou E. Volatile compounds from the red deer (Cervus elaphus). Substances secreted via the urine. Comparative Biochemistry and Physiology. 1990;97A:427–431. [Google Scholar]
- Barsan C, Sanchez-Bel P, Rombaldi C, Egea I, Rossignol M, Kuntz M, Zouine M, Latché A, Bouzayen M, Pech JC. Characteristics of the tomato chromoplast revealed by proteomic analysis. Journal of Experimental Botany. 2010;61:2413–2431. doi: 10.1093/jxb/erq070. [DOI] [PubMed] [Google Scholar]
- Bathgate B, Purton ME, Grierson D, Goodenough PW. Plastid changes during the conversion of chloroplasts to chromoplasts in ripening tomatoes. Planta. 1985;165:197–204. doi: 10.1007/BF00395042. [DOI] [PubMed] [Google Scholar]
- Bian W, Barsan C, Egea I, Purgatto E, Chervin C, Zouine M, Latché A, Bouzayen M, Pech J-C. Metabolic and molecular events occurring during chromoplast biogenesis. Journal of Botany. 2011:Article ID 289859, 13. [Google Scholar]
- Bold HC. The nutrition of the sporophyte in the Musci. American Journal of Botany. 1940;27:318–322. [Google Scholar]
- Dodge JD. Changes in chloroplast fine structure during the autumnal senescence of Betula leaves. Annals of Botany. 1970;34:817–824. [Google Scholar]
- Hartvigsen K, Lund P, Hansen LF, Hølmer G. Dynamic headspace gas chromatography/mass spectrometry characterization of volatiles produced in fish oil enriched mayonnaise during storage. Journal of Agricultural and Food Chemistry. 2000;48:4858–4867. doi: 10.1021/jf991385b. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Nishimura M. Frontiers of research on organelle differentiation. Plant and Cell Physiology. 2009;50:1995–1999. doi: 10.1093/pcp/pcp161. [DOI] [PubMed] [Google Scholar]
- Inaba T, Ito-Inaba Y. Versatile roles of plastids in plant growth and development. Plant and Cell Physiology. 2010;51:1847–1853. doi: 10.1093/pcp/pcq147. [DOI] [PubMed] [Google Scholar]
- Jürgens A, Dötterl S, Meve U. The chemical nature of fetid floral odours in stapeliads (Apocynaceae-Asclepiadoideae-Ceropegieae) New Phytologist. 2006;172:452–468. doi: 10.1111/j.1469-8137.2006.01845.x. [DOI] [PubMed] [Google Scholar]
- Jüttner F, Watson SB, von Elert E, Köster O. β-Cyclocitral, a grazer defence signal unique to the cyanobacterium Microcystis. Journal of Chemical Ecology. 2010;36:1387–1397. doi: 10.1007/s10886-010-9877-0. [DOI] [PubMed] [Google Scholar]
- Karunen P. Polyunsaturated hydrocarbons from Polytrichum commune spores. Phytochemistry. 1974;13:2309–2313. [Google Scholar]
- Kishimoto K, Matsui K, Ozawa R, Takabayashi J. Volatile 1-octen-3-ol induces a defensive response in Arabidopsis thaliana. Journal of General Plant Pathology. 2007;73:35–37. [Google Scholar]
- Koponen A. Entomophily in the Splachnaceae. Botanical Journal of the Linnean Society. 1990;104:115–127. [Google Scholar]
- Lewinsohn E, Sitrit Y, Bar E, Azulay Y, Ibdah M, Meir A, Yosef E, Zamir D, Tadmor Y. Not just colors – carotenoid degradation as a link between pigmentation and aroma in tomato and watermelon fruit. Trends in Food Science and Technology. 2005;16:407–415. [Google Scholar]
- Majetic C, Rausher MD, Raguso RA. The pigment–scent connection: do mutations in regulatory vs. structural anthocyanin genes differentially alter floral scent production in Ipomoea purpurea? South African Journal of Botany. 2010;76:632–642. [Google Scholar]
- Marino PC. Dispersal and coexistence of mosses (Splachnaceae) in patchy habitats. Journal of Ecology. 1991;79:1047–1060. [Google Scholar]
- Marino P, Raguso R, Goffinet B. The ecology and evolution of fly dispersed dung mosses (Family Splachnaceae): manipulating insect behaviour through visual and odour cues. Symbiosis. 2009;47:61–76. [Google Scholar]
- Morita MT. Directional gravity sensing in gravitropism. Annual Review of Plant Biology. 2010;61:705–720. doi: 10.1146/annurev.arplant.043008.092042. [DOI] [PubMed] [Google Scholar]
- Morita MT, Tasaka M. Gravity sensing and signaling. Current Opinion in Plant Biology. 2004;7:712–718. doi: 10.1016/j.pbi.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Pyysalo H, Koponen A, Koponen T. Studies on entomophily in the Splachnaceae (Musci). I. Volatile compounds in the sporophyte. Annales Botanici Fennici. 1978;15:293–296. [Google Scholar]
- Pyysalo H, Koponen A, Koponen T. Studies on entomophily in the Splachnaceae (Musci). II. Volatile compounds in the hypophysis. Annales Botanici Fennici. 1983;20:335–338. [Google Scholar]
- Renault S, Bonnemain JL, Faye L, Gaudillere JP. Physiological aspects of sugar exchange between the gametophyte and sporophyte of Polytrichum formosum. Plant Physiology. 1992;100:1815–1822. doi: 10.1104/pp.100.4.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack FD, Schwuchow JM, Wagner T, Kern V. Gravity sensing in moss protonemata. Advances in Space Research. 2001;27:871–876. doi: 10.1016/s0273-1177(01)00151-x. [DOI] [PubMed] [Google Scholar]
- Sagisaka S. The proliferation of amyloplasts in meristematic cells of developing stolons of potato and apple callus: progenitors of proplastids. Journal of Plant Physiology. 2008;165:1678–1690. doi: 10.1016/j.jplph.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Tellez MR, Schrader KK, Kobaisy M. Volatile components of the cyanobacterium Oscillatoria perornata (Skuja) Journal of Agricultural and Food Chemistry. 2001;49:5989–5992. doi: 10.1021/jf010722p. [DOI] [PubMed] [Google Scholar]
- Thompson JE, Froese CD, Madey E, Smith MD, Hong Y. Lipid metabolism during plant senescence. Progress in Lipid Research. 1998;37:119–141. doi: 10.1016/s0163-7827(98)00006-x. [DOI] [PubMed] [Google Scholar]
- Tieman DM, Zeigler M, Schmelz EA, Taylor MG, Bliss P, Kirst M, Klee HJ. Identification of loci affecting flavour volatile emissions in tomato fruits. Journal of Experimental Botany. 2006;57:887–906. doi: 10.1093/jxb/erj074. [DOI] [PubMed] [Google Scholar]
- Troilo D, Cameron RG. Comparative behavior of Pyrellia cyanicolor (Diptera: Muscidae) on the moss Splachnum ampullaceum and on substrates of nutritional value. Great Lakes Entomologist. 1981;14:191–195. [Google Scholar]
- Vogel S. The role of scent glands in pollination: on the structure and function of osmophores. Smithsonian Institution Libraries; Washington, DC, USA: 1990. p. 202. [Google Scholar]
- Walter MH, Floss DS, Strack D. Apocarotenoids: hormones, mycorrhizal metabolites and aroma volatiles. Planta. 2010;232:1–7. doi: 10.1007/s00425-010-1156-3. [DOI] [PubMed] [Google Scholar]
- Whittle CL, Bowyer RT, Clausen TP, Duffy LK. Putative pheromones in urine of rutting male moose (Alces alces): evolution of honest advertisement? Journal of Chemical Ecology. 2000;26:2747–2762. [Google Scholar]
- Zhang B, Yin XR, Li X, Yang SL, Ferguson IB, Chen KS. Lipoxygenase gene expression in ripening kiwifruit in relation to ethylene and aroma production. Journal of Agricultural and Food Chemistry. 2009;57:2875–2881. doi: 10.1021/jf9000378. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SPME-GC-MS analyses and identity of 69 VOC from whole or dissected tissues of S. ampullaceum.
Summary of GC-MS data for dissected sporophytes and spores.