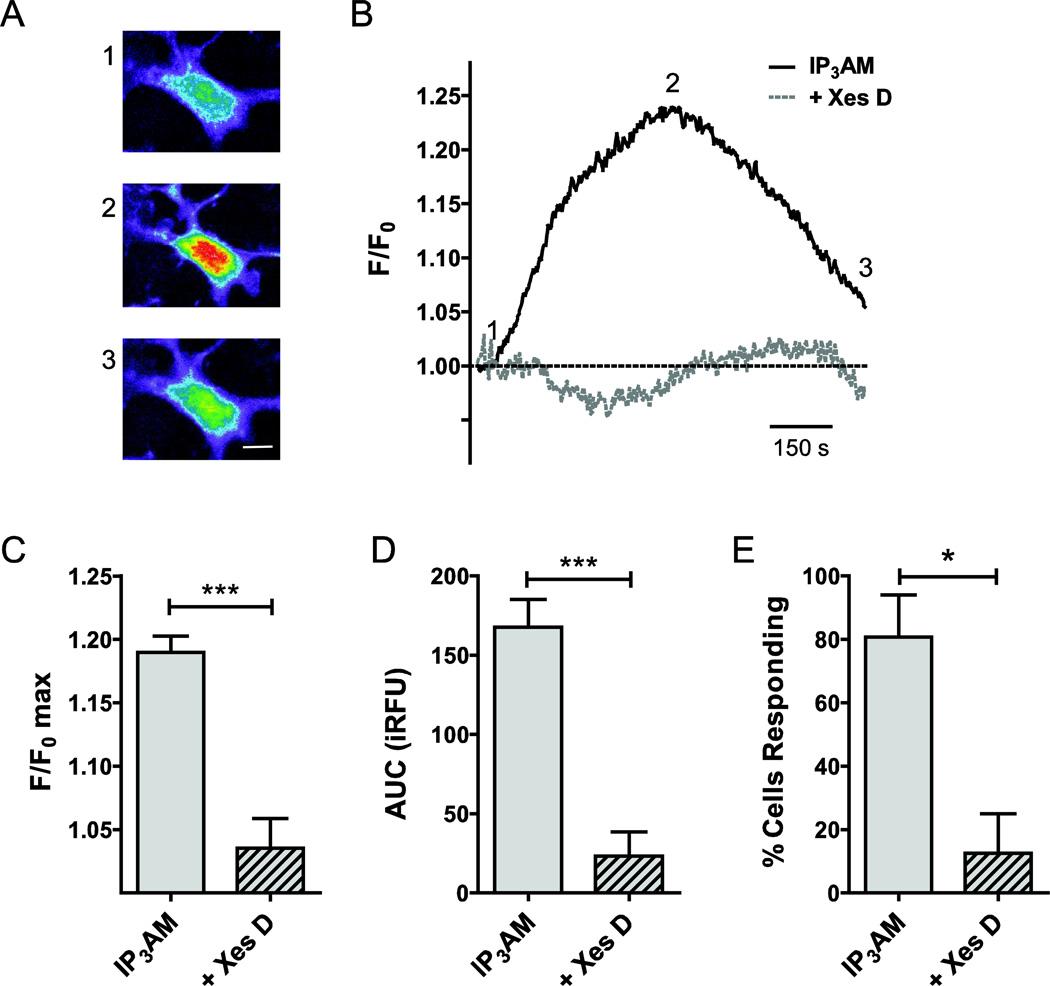
Figure 4. Functional contributions of IP3Rs to intracellular Ca2+ release in primary cultured rat ONHAs.
In order to determine the contribution of IP3Rs to Ca2+ release from intracellular stores, we quantified the rise in intracellular Ca2+ concentration upon stimulation with the IP3R-selective agonist, IP3, using optical imaging and the Ca2+ fluorophore, Fura-2. For delivery, we used the membrane-permeable molecules IP3-AM (10 µM) and Fura-2-AM (1 µM). Representative cellular responses (A) and traces (B) are shown. Warmer colors indicate higher fluorescence intensity. IP3 stimulation resulted in a mean 19.0 ± 1.3% increase of normalized fluorescence intensity over baseline (F/F0 max; n=47; C). This corresponded to an experimentally determined AUC of 168 ± 18 iRFU (n=45; D). In our experiments, 81 ± 13% of cells responded to IP3 stimulation (n=3 separate experiments, with at least 15 cells per experiment; E). In order to determine the specificity of the response, we pre-incubated cell with the IP3R blocker, Xestospongin D (Xes D), for 30 min, and experiments performed with Xes D co-application. Xes D resulted in a 15.4 ± 2.5% reduction of F/F0 max (n=18, P<0.001; C), and reduced AUC significantly by 145 ± 30 iRFU (n=18, P<0.001; D). The number of cells responding to IP3 in the presence of Xes D was reduced significantly by 68% (P<0.05; E). * P<0.05; *** P<0.001. Scale bar: 10 µm.