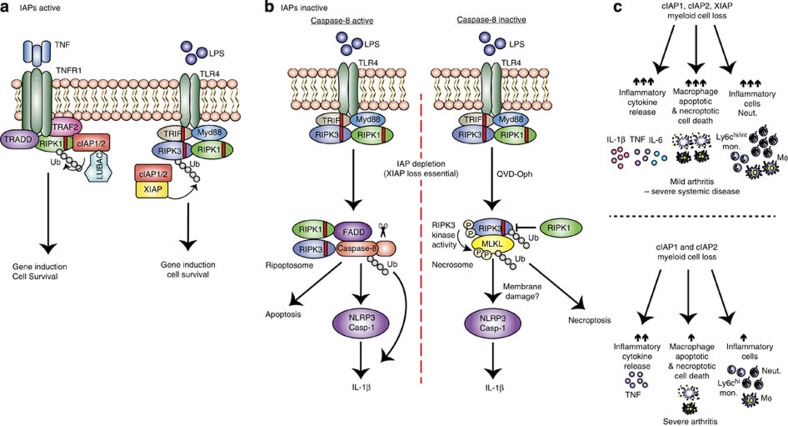
Figure 10. Model for how XIAP and cIAPs repress inflammatory cytokine production, apoptosis and necroptosis.
(a) When IAPs are present, TNFR1 or TLR–TRIF signalling results in IAP-mediated ubiquitylation of RIPK1 or RIPK3, respectively, to propagate pro-survival signals and gene induction. (b) If IAPs are inactivated but caspase-8 is present (left panel), LPS stimulation induces the ripoptosome platform to activate caspase-8 (ubiquitylated). Caspase-8 can (i) trigger apoptosis, (ii) cleave pro-IL-1β directly into its mature form or (iii) promote NLRP3-associated caspase-1 activation, by a mechanism yet to be defined. Alternatively, if both IAPs and caspase-8 are inactive (right panel), LPS induces the formation of the RIPK3–MLKL necrosome that, in addition to causing necroptotic cell death, activates the NLRP3 inflammasome. This necroptotic pathway is associated with RIPK3 and MLKL ubiquitylation, which may control RIPK3/MLKL signalling and/or stability. (c) Genetic loss or inhibition of cIAPs alone or XIAP and cIAPs in myeloid cells cause differential effects on cell death, cytokine production and haematopoiesis leading to spontaneous arthritis. Loss of all three IAPs leads to spontaneous systemic inflammatory disease, featuring mild joint inflammation. Disease is associated with increased cytokine release, including IL-1β and TNF, as well as apoptotic and necroptotic cell death and the accumulation of innate inflammatory cells. In contrast, loss of cIAP1/2 causes severe arthritis that is associated specifically with enhanced TNF levels and only modest effects on haematopoiesis.