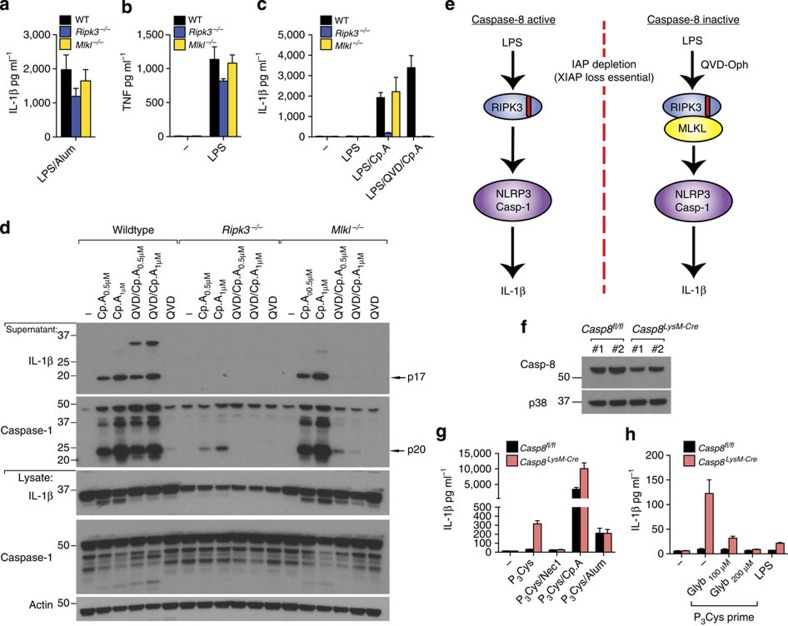
Figure 3. RIPK3 activates caspase-1 independent of MLKL unless caspase-8 is inhibited.
(a–c) WT, Mlkl−/− and Ripk3−/− BMDM were primed with LPS (20 ng ml−1) for 3 h and cultured with Q-VD-OPh (20 μM), where indicated, which was added in the last 20 min of priming. Cells were then stimulated with Cp.A (500 nM) or alum (300 μg ml−1) for a further 6 h. Supernatants were analyzed for (a,c) IL-1β and (b) TNF by ELISA. n=3 mice per genotype. Data are represented as mean+s.e.m. and are representative of one of three independent experiments. (d) WT, Mlkl−/− and Ripk3−/− BMDM were primed with LPS for 2.5 h. In the last 20 min of priming, cells were incubated with Q-VD-OPh (20 μM) and then cultured with Cp.A (1 μM) for 5 h. Cell supernatants and lysates were analyzed by immunoblot. Representative of one of three experiments. Full-size immunoblots are presented in Supplementary Fig. 11. (e) Schematic depicting how RIPK3 signals IL-1β activation based on the data presented in Figs 1, 2, 3. (f) Lysates from WT (Casp8fl/fl) littermate and caspase-8-deficient (Casp8LysMcre) BMDM (n=2 mice) were subjected to immunoblot to assess efficiency of caspase-8 deletion. Full-size immunoblots are presented in Supplementary Fig. 11. (g) WT littermate and Caspase-8LysMcre BMDM were primed for 3 h with Pam3Cys (2 μg ml−1), and as indicated treated with Nec-1 (50 μM) in the last 20 min of priming. Cells were then exposed to Cp.A (500 nM), as specified, for a further 24 h, after which IL-1β release was measured by ELISA. n=3 mice per genotype, mean+s.e.m. Representative of one of three experiments. (h) WT littermate and Caspase-8LysMcre BMDM were pre-incubated with glyburide for 20 min, as indicated, and cultured with Pam3Cys (2 μg ml−1) or LPS (100 ng ml−1) for 24 h. Cell supernatants were assayed for IL-1β by ELISA. n=4 mice per genotype, mean+s.e.m. Representative of one of two experiments.