Abstract
Kidney fibrosis is the final common pathway for virtually every type of chronic kidney disease and is a consequence of a prolonged healing response that follows tissue inflammation. Chronic kidney inflammation ultimately leads to progressive tissue injury and scarring/fibrosis. Several pathways have been implicated in the progression of kidney fibrosis. In the present study, we demonstrate that G protein-coupled chemokine (C-X-C motif) receptor (CXCR)4 was significantly upregulated after renal injury and that sustained activation of Cxcr4 expression augmented the fibrotic response. We demonstrate that after unilateral ureteral obstruction (UUO), both gene and protein expression of Cxcr4 were highly upregulated in tubular cells of the nephron. The increased Cxcr4 expression in tubules correlated with their increased dedifferentiated state, leading to increased mRNA expression of platelet-derived growth factor (PDGF)-α, transforming growth factor (TGF)-β1, and concurrent loss of bone morphogenetic protein 7 (Bmp7). Ablation of tubular Cxcr4 attenuated UUO-mediated fibrotic responses, which correlated with a significant reduction in PDGF-α and TGF-β1 levels and preservation of Bmp7 expression after UUO. Furthermore, Cxcr4+ immune cells infiltrated the obstructed kidney and further upregulate their Cxcr4 expression. Genetic ablation of Cxcr4 from macrophages was protective against UUO-induced fibrosis. There was also reduced total kidney TGF-β1, which correlated with reduced Smad activation and α-smooth muscle actin levels. We conclude that chronic high Cxcr4 expression in multiple effector cell types can contribute to the pathogenesis of renal fibrosis by altering their biological profile. This study uncovered a novel cross-talk between Cxcr4-TGF-β1 and Bmp7 pathways and may provide novel targets for interrupting the progression of fibrosis.
Keywords: chemokine (C-X-C motif) receptor 4, AMD-3100, kidney, fibrosis, macrophages, bone morphogenetic protein 7
kidney fibrosis is the end result for virtually every type of chronic kidney disease (CKD). Fibrosis is the result of healing response that follows tissue inflammation. Tissue inflammation may be the result of simple wounding or chronic inflammation, which, in the case of the kidney, leads to scarring. Kidney fibrosis consists of glomerular, vascular sclerosis, and tubulointerstitial fibrosis. The histological picture of tubulointerstitial fibrosis is characterized by tubular atrophy, tubular dilatation, interstitial leukocyte infiltration, fibroblast accumulation, vascular rarefaction, and continuous deposition of matrix proteins (66). Although substantial progress has been made in our understanding of the processes that lead to fibrosis, a viable therapy is still not available. Multiple laboratories have identified multiple effectors that, after an injury, can contribute to the process of fibrosis. These include tubular epithelial cells, endothelial cells, leukocytes, and, more recently, pericytes (27, 39, 47, 48, 84, 87, 89, 90). Injured tubular cells secrete cytokines and chemokines.
Chemokines are a family of small secreted proteins that induce a chemotactic response in cells expressing the appropriate chemokine receptor. Chemokine receptors were first identified on leukocytes, but we now know that nonhematopoietic cells too express various chemokine receptors (49). Chemokine receptors are seven-transmembrane domain G protein-coupled cell surface receptors. The interaction between a chemokine and its receptor(s) not only coordinates trafficking of immune cells to specific tissue location but may also play an important role in pathological conditions such as metastasis in certain cancers. Chemokine receptors are designated as chemokine (C-X-C motif) receptor (CXCR)1 to CXCR5, chemokine (C-C motif) receptor (CCR)1 to CCR11, and chemokine (C-X3-C motif) receptor 1. In the present study, we determined the role of CXCR4 in kidney fibrosis.
Cxcr4 is ubiquitously expressed and has a single known ligand, stromal cell-derived factor (Sdf)-1α, which is also known as chemokine (C-X-C motif) ligand 12 (67). Cxcr4 is highly expressed in the embryonic kidney, but expression is significantly low in the adult kidney (68, 76). We have previously demonstrated the importance of Cxcr4 signaling in normal branching morphogenesis of the ureteric bud during early kidney development (76). After injury (such as ischemic injury), in mice, the tubular expression of Cxcr4 is transiently upregulated, perhaps as a reparative signal (72). Intense Cxcr4 staining is also observed in distal and proximal tubules of immunostained human biopsies of IgA nephropathy, minimal-change nephrotic syndrome, focal segmental glomerulosclerosis, chronic pyelonephritis, and acute tubular necrosis. In addtion, varying degrees of CD45+ Cxcr4+ infiltrates are seen in these biopsies, which also correlated with intense staining for Sdf-1α (50). Thus, there seems to be an association between high Cxcr4 expression and kidney disease.
In certain cancers, chronic high Cxcr4 expression induces mesenchymal-like characteristics in epithelial cells by activating matrix metalloproteinase-2 and -9 (16, 81), which affect matrix remodeling.
Chronic inflammation in the kidney creates a milieu for maintaining high Cxcr4 expression. One such stimulus is localized hypoxia (due to vascular rarefaction), which stimulates the expression of transcription factors like hypoxia-inducible factor-1α and Foxc1/2, in addition to the cytokine transforming growth factor (TGF)-β1, all of which are known to stimulate Cxcr4 mRNA expression (6, 8, 15, 36, 56, 72). Upon binding to Sdf-1, CXCR4 initiates an intracellular signaling cascade resulting in cell type- and context-specific responses (8, 76). We have previously reported that Cxcr4 activates MAPK1/2, phosphatidylinositol 3-kinase (PI3K), and PKC signaling in tubular epithelial cells (76). It also activates STAT- and NF-κB-dependent signaling pathways in other cell types (28, 34, 43, 51, 70). The transcriptional targets downstream of Cxcr4 signaling are not fully known. However, a recent study (1) determined the transcription factor profiles of Cxcr4-high and Cxcr4-low subpopulations of MDA-MB-231 cells, a breast cancer cell line. The authors determined that high CXCR4 expression led to differential expression of ∼200 genes (1).
A prominent feature in biopsy specimens of CKD patients is that of significant macrophage infiltration, which correlates directly with interstitial fibrosis and inversely with prognosis (24, 25, 86). Macrophage ablation in mice attenuates fibrosis and restores tubular health (23, 40). However, macrophages can switch between proinflammatory and anti-inflammatory (prorepair/profibrotic) phenotypes depending on the local microenvironment. Proinflammatory phenotypes are characterized by the secretion of IL-1β, IL-12, and TNF-α, whereas exposure to IL-4 induces a profibrotic program characterized by expression of scavenger receptors [macrophage scavenger receptor (Msr)1], mannose receptor C type 1 (Mrc1), and expression of profibrotic mediators such as arginase-1 (in mice) and Fizz-1 (in mice and humans), among others. Interestingly, profibrotic M2 macrophages express 16-fold greater Cxcr4 mRNA levels in human peripheral blood-derived macrophages (53). Its significance is not known. Increased Cxcr4 expression is observed in leukocytes from multiple murine models of lupus nephritis, where heightened Toll-like receptor and cytokine signaling accounted for part of this increased expression. Treatment of these mice with a peptide antagonist of Cxcr4 ameliorated leukocyte trafficking and end-organ disease in these models (79). We thus hypothesized that the milieu in a chronically injured kidney would induce sustained Cxcr4 expression in multiple cell types. Sustained high Cxcr4 expression (on tubules or immune cells, such as macrophages), in turn, will exacerbate renal injury and thereby promote fibrosis. Therefore, interrupting this pathway should ameliorate fibrosis by acting upon multiple effector cells, such as tubular and infiltrating lymphoid cells. We tested this hypothesis in the mouse unilateral ureteral obstruction (UUO) model of renal fibrosis.
In the present study, we demonstrate that Cxcr4 expression in the kidney was upregulated in tubular and infiltrating immune cells after UUO. Mice administered the CXCR4 antagonist AMD-3100 (AMD) had partial protection from UUO-induced fibrosis and a modest but significant reduction in macrophage infiltration. Mice lacking Cxcr4, specifically in the nephron, developed significantly less fibrosis upon ureteral ligation. Ablation of Cxcr4 selectively in macrophages significantly blunted UUO-induced fibrosis. Interruption of the Cxcr4 pathway positively upregulated bone morphogenetic protein 7 (Bmp7) while negatively modulating TGF-β1 expression. The data suggest that chronic kidney injury results in sustained high levels of Cxcr4 in multiple effector cells that can contribute to the progression of fibrosis. Identifying downstream effectors of this pathway could provide new therapeutic targets for treating kidney fibrosis.
MATERIALS AND METHODS
Animal Models
All experiments involving animals were performed as per approved guidelines of the Institutional Animal Care and Use Committee of Yale University. Bmp7-Cre mice (a gift from Dr. Oxburgh, Maine Medical Center Research Institute) and LysM-Cre mice (JAX Labs) were bred with Cxcr4fl/fl mice (a gift from Dr. Miller, Northwestern University, Chicago, IL). All mice were on the C57Bl/6 background.
UUO
Cxcr4fl/fl, Bmp7-Cre;Cxcr4fl/fl, and LysM-Cre;Cxcr4fl/fl mice (male, age: 6–8 wk old) were anesthetized with intraperitoneal ketamine (100 mg/kg) and xylazine (20 mg/kg) followed by subcutaneous injection of an analgesic (0.05 mg/kg buprenorphine) before incision. A lateral flank incision was made, and the left ureter was double ligated using 3-0 silk. For treatment with the Cxcr4 antagonist AMD, 5 mice/group were distributed into the following three treatment groups: saline (vehicle), AMD (5 mg/kg labeled as AMD5 group), and AMD (10 mg/kg labeled as AMD10 group). AMD or saline was injected subcutaneously every 12 h. Mice were euthanized between days 6 and 12 after ureter ligation. Mice were perfused with saline, and kidneys were dissected out for the following analyses.
Histology and Immunostaining
Kidneys were processed for hematoxylin and eosin staining and Masson's trichrome staining by the Histopathology Core at Yale School of Medicine. Cxcr4 immunostaining was performed using anti-Cxcr4 antibody (clone 2B11, eBiosciences, at 1:50 dilution overnight) and the appropriate secondary antibody at 1:200 dilution using standard immunohistochemistry procedures. Collagen type I (Col1) antibody was a kind gift from Dr. Joseph Madri (Yale School of Medicine). Staining was analyzed using a Nikon Eclipse TE2000-U microscope, and images taken with a ×4–40 objective and processed in Adobe Photoshop CS. A renal pathologist (G. Moeckel) masked to the identity of the animal scored each kidney specimen for fibrosis using recently described protocol. Respective kidney sections were scored for tubular injury by a blinded renal pathologist. Scoring was carried out by calculating the percentage of tubules in the cortex that displayed cell necrosis, loss of the brush border, and a dilated lumen as follows: 0 = none, 1 = 10%, 2: 11–25%, 3 = 26–45%, 4 = 46–75%, and 5 = 75–100%. Ten randomly chosen fields were examined for each slide (13, 55).
FACS analysis.
Kidneys were dissected, minced, and collected in 400 μl Liberase (catalog no. 05-401-119-001, Roche Diagnostics) containing DNAse-I (Sigma-Aldrich, St. Louis, MO). Kidneys were digested for 60 min at 37°C in an orbital shaker. The digest was then passed through an 18-gauge needle onto a 40-μm membrane filter to make a single cell suspension. Cells were suspended with staining buffer (1% FBS in PBS) and stained with the following antibodies: anti-Cxcr4-conjugated AF647 (catalog no. 51-9991-80, eBiosciences), isotype control (catalog no. 557690, BD Biosciences, San Jose, CA), anti-F4/80 (FITC conjugated, catalog no. 11-4801-82, eBiosciences), anti-CD45 (PERCP conjugated, catalog no. 557235, BD Biosciences), and anti-lymphocyte antigen (Ly)6C, anti-CD3, anti-Gr1, and anti-Cd11C (BD Biosciences). Cells were analyzed using a LSRII analyzer (BD Biosciences), and data were analyzed using FloJo software (Tree Star, OR). Cells were sorted using Beckman Coulter MoFlo (Brea, CA).
Immunoblot analysis.
Whole kidney lysates were prepared by homogenization in ice-cold Pierce IP lysis buffer containing Halt protease and phosphatase inhibitor cocktail (Thermo Fisher scientific, Rockford, IL). Lysates were centrifuged (14,000 g for 20 min at 4°C), and the supernatant was collected. Protein concentration was determined using a Bradford assay. Equal amounts of protein were subjected to SDS-PAGE analysis on precast polyacrylamide 4–15% gradient gels (Bio-Rad) and transferred to polyvinylidene difluoride membranes (Millipore). Membranes were blocked in 5% milk with Tris-buffered saline-Tween and incubated overnight at 4°C with the following primary antibodies: Cxcr4 (BD Biosciences), α-smooth muscle actin (α-SMA), fibroblast-specific protein-1 (Fsp1), vimentin, E-cadherin, GAPDH, β-actin (Santa Cruz Biotechnology, Santa Cruz, CA), phosphorylated (p)Smad2/pSmad3 (D27F4, Cell Signaling, Danvers, MA), and TGF-β1 (R&D Systems, Minneapolis, MN).
mRNA analysis.
After surgical removal and homogenization of the kidneys, RNA was isolated using RNA-STAT60 (Tel-Test, Friendswood, TX) or RNAeasy mini kit (Qiagen, Germantown, MD), and 1 μg was subsequently transcribed into cDNA using Iscript (Bio-Rad). A 1:10 dilution of this cDNA was used for quantitative real-time PCR analysis using iTaq Universal SYBR Green Supermix (Bio-Rad). Primers for each analysis were optimized beforehand. A Bio-Rad CFX-96 Real-Time PCR machine was used to carry out the reaction and analysis of cycle time (Ct) values. The respective Ct values were normalized to hypoxanthine phosphoribosyltransferase 1, and values from all experimental groups were expressed as relative to the uninjured kidney or vehicle control for in vitro experiments. Primers for Cxcr4, Sdf-1α, Col1a, and collagen type III (Col3)a were purchased from SA Biosciences (Valencia, CA). The primer sequences for the rest of the genes are shown in Table 1.
Table 1.
Primer list
| Hypoxanthine phosphoribosyltransferase 1 | |
| Forward | 5′-CAGTACAGCCCCAAAATGGT-3′ |
| Reverse | 5′-CAAGGGCATATCCAACAACA-3′ |
| IL-1 β | |
| Forward | 5′-TGTGAAATGCCACCTTTTGA-3′ |
| Reverse | 5′-TGTCCTCATCCTGGAAGGTC-3′ |
| IL-4 | |
| Forward | 5′-TCAACCCCCAGCTAGTTGTC-3′ |
| Reverse | 5′-TGTTCTTCGTTGCTGTGAGG-3′ |
| IL-6 | |
| Forward | 5′-AACGATGATGCACTTGCAGA-3′ |
| Reverse | 5′-GGAAATTGGGGTAGGAAGGA-3′ |
| IL-10 | |
| Forward | 5′-CCAGTTTTACCTGGTAGAAGTGATG-3′ |
| Reverse | 5′-TGTCTAGGTCCTGGAGTCCAGCAGACTC-3′ |
| Macrophage scavenger receptor 1 | |
| Forward | 5′-AAAGGGAGAGAAGGGGAGTG-3′ |
| Reverse | 5′-GCATGACACAGGAACCAATG-3′ |
| Mannose receptor C type 1 | |
| Forward | 5′-CAAGGAAGGTTGGCATTTGT-3′ |
| Reverse | 5′-CCTTTCAGTCCTTTGCAAGC-3′ |
| Connective tissue growth factor | |
| Forward | 5′-AGCAGCTGGGAGAACTGTGT-3′ |
| Reverse | 5′-TGGTATTTGCAGCTGCTTTG-3′ |
| Bone morphogenetic factor 7 | |
| Forward | 5′-ACAAGGCCGTCTTCAGTACC-3′ |
| Reverse | 5′-CGCTCCCGGATGTAGTCC-3′ |
| Monocyte chemotactic protein-1 | |
| Forward | 5′-AGGTCCCTGTCATGCTTCTG-3′ |
| Reverse | 5′-TCTGGACCCATTCCTTCTTG-3′ |
| Collagen type IVA1 | |
| Forward | 5′-CTGGAGAAAAGGGCCAGAT-3′ |
| Reverse | 5′-TCCTTAACTTGTGCCTGTCCA-3′ |
| α-Smooth muscle actin | |
| Forward | 5′-ACTGGGACGACATGGAAAAG-3′ |
| Reverse | 5′-CATCTCCAGAGTCCAGCACA-3′ |
| Fibronectin | |
| Forward | 5′-ATGACGATGGGAAGACCTAC-3′ |
| Reverse | 5′-GAAGCACTCAATGGGGCAAAT-3′ |
| TNF-α | |
| Forward | 5′-GAACTGGCAGAAGAGGCACT-3′ |
| Reverse | 5′-AGGGTCTGGGCCATAGAACT-3′ |
| Fibroblast-specific protein-1 | |
| Forward | 5′-TTGTGTCCACCTTCCACAAA-3′ |
| Reverse | 5′-GCTGTCCAAGTTGCTCATCA-3′ |
| Transforming growth factor-β1 | |
| Forward | 5′-TGAGTGGCTGTCTTTTGACG-3′ |
| Reverse | 5′-GGTTCATGTCATGGATGGTG-3′ |
| Platelet-derived growth factor-α | |
| Forward | 5′-GGTACTGAATTTCGCCGCCA-3′ |
| Reverse | 5′-GGTCTGGGTTCAGGTTGGAG-3′ |
| Arginase-1 | |
| Forward | 5′-CGACCCAAGAAGACTAGAGCC-3′ |
| Reverse | 5′-CTCGCAAGCCAATGTACACG-3′ |
| Inducible nitric oxide synthase | |
| Forward | 5′-GGGTCACAACTTTACAGGGAGT-3′ |
| Reverse | 5′-CTCTCCACTGCCCCAGTTTT-3′ |
Bone Marrow Macrophage Isolation and Culture
Six-week-old C57BL/6 mice were euthanized by injection of a lethal dose of ketamine and xylazine. Femurs and tibias were collected, and the marrow was flushed from each bone into a Falcon tube using a 25-gauge needle and 12-ml syringe filled with α-MEM. The marrow was homogenized and passed through a 40-μm cell strainer before centrifugation at 12,000 rpm for 10 min. The supernatant was discarded, and the pellet was suspended in 3 ml of 1× cell lysis buffer for 2 min, after which 27 ml α-MEM was added. The mixture was then centrifuged at 12,000 rpm for another 10 min. Cells were suspended and plated at 1 × 106 cells/ml in α-MEM containing 10% L929, 10% FBS, 1% glutamine, and 1% vitamins and cultured at 37°C and 5% CO2. The supernatant was removed from the dishes after 18 h, and cells were replated at the same concentration in new medium. Seventy-two hours later, 5 ml α-MEM containing 30% L929, 10% FBS, 1% glutamine, and 1% vitamins was added to each dish. For determining signaling or changes in gene expression after stimulation with Sdf-1, cells were switched to α-MEM + ITS (Sigma). Cells were stimulated with Sdf-1 or vehicle (PBS) for the times mentioned in the figures.
Isolation and FACS Sorting of Kidney Macrophages and Tubular Cells
Macrophages were isolated from uninjured and UUO kidney single cell suspensions at the defined time points, as previously described by our laboratory (41). Cells stained for macrophage markers were sorted by a Beckman Coulter MoFlo. Total RNA was isolated and processed to determine gene expression by quantitative real-time PCR. To sort tubular cells, the kidney cell suspension was labeled with collecting duct- and proximal tubule-specific lectins (Dolichos biflorus agglutinin and Lotus tetragonolobus, respectively, 1:50 dilution, Vector Laboratories, Burlingame, CA) and FACS sorted. The two fractions were pooled together for expression experiments.
Transduction of Cxcr4
Retroviral transduction of human kidney proximal tubule cells (HKC2 cells) with human Cxcr4 was performed as previously described (5). Quantitative RT-PCR, Western blot analysis, and FACS were used to confirm the overexpression of Cxcr4. Cells from three separate transductions were pooled together to avoid any clonal effects and variations.
Statistical Analysis
A two-tailed t-test and one-way ANOVA were used to compare data between groups using Prism 5.0 (GraphPad Software, La Jolla, CA). Significance was determined at P < 0.05. Data are presented as means ± SE.
RESULTS
The Total Cxcr4 Content of the Kidney Increases After UUO
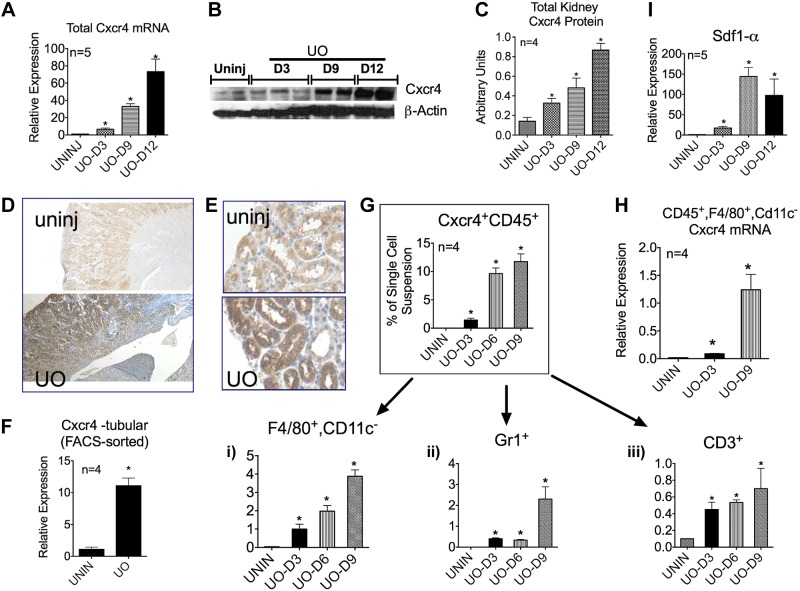
Total Cxcr4 mRNA and protein content of the kidneys were measured on days 3, 9, and 12 of UUO and compared with those of uninjured kidneys. As hypothesized, a gradual and significant increase in Cxcr4 content was observed after UUO. Compared with uninjured kidneys, day 12 UUO kidneys had an ∼70-fold increase in total Cxcr4 mRNA content (n = 5; Fig. 1A). This correlated with a corresponding increase in Cxcr4 protein content, as shown in a representative immunoblot (Fig. 1B) and quantified from a total of 4 mice/time point (Fig. 1C). Consistent with the reported increase in chemotactic Cxcr4 ligand Sdf-1α after renal ischemic (73), we observed a significant increase in Sdf-1α mRNA content in UUO kidneys by day 9 (Fig. 1I).
Fig. 1.
After unilateral ureteral obstruction (UUO or UO), chemokine (C-X-C motif) receptor (Cxcr)4 expression increased in tubules and macrophages, and Cxcr4+ immune cells accumulated in obstructed kidneys. A: total kidney Cxcr4 mRNA content was measured by quantitative RT-PCR on days (D)3, 9, and 12 of UUO and compared with uninjured (uninj) kidneys (n = 5). B and C: total kidney Cxcr4 protein content was analyzed by Western blot analysis. B: representative blot; C: densitometry from two blots (n = 4). D and E: paraffin-embedded tissue sections from uninjured and day 9 UUO kidneys were immunostained for Cxcr4. Representative pictures (magnification: ×100; D) and (magnification × 400; E) are shown. F: Cxcr4 mRNA expression on lectin-labeled tubular cells FACS sorted from uninjured and UUO kidneys (n = 4). G: single cell suspensions from the respective kidneys were analyzed for Cxcr4+ CD45+ cells by FACS, and percentage of macrophages (i), granulocytes (ii), and CD3+ T cells (iii) were determined. H: Cxcr4 mRNA expression on macrophages in UUO kidneys increased with time (n = 4). I: mRNA content of stromal-derived factor (Sdf)-1α also increased severalfold from days 3 to 12 of UO (n = 5). *P < 0.05.
Tubular and Immune Cells Contribute to the Increased Cxcr4 Content Observed in UUO Kidneys
To determine the source of increased Cxcr4 mRNA expression, we performed immunohistochemistry and FACS sorted proximal tubule cells, collecting duct cells, and the CD45+ myeloid cell population from uninjured and day 6 UUO kidneys. Cxcr4 expression in an adult healthy kidney is known to be low (50, 68, 76), which was confirmed by immunohistochemistry of the uninjured kidney (Fig. 1, D and E). After UUO, a generalized but significant increase in Cxcr4 staining was observed in the renal parenchyma. Furthermore, mRNA analysis of FACS-sorted tubular cells from day 6 UUO kidneys revealed an ∼10-fold increase in Cxcr4 mRNA compared with cells from uninjured kidneys (Fig. 1F).
Using FACS analysis, we also detected a progressive accumulation of Cxcr4+ CD45+ cells in obstructed kidneys from days 3 to 12 of UUO (Fig. 1G). This is in agreement with what has been previously reported after ischemia-reperfusion and at the sites of tissue inflammation (3, 73, 83). Further analysis of this Cxcr4+ CD45+ cell population revealed that by day 9 of UUO, 40% were F4/80+ Cd11clo macrophages, ∼20% were Gr1+ (immature monocytes and/or granulocytes), and ∼1% were CD3+ T cells (Fig. 1G,i–iii). Interestingly, similar to tubular cells, the Cxcr4 mRNA content of F4/80+ Cd11clo cells too increased gradually from days 3 to 9 of UUO. Taken together, these data suggest that the total increase in Cxcr4 content observed after UUO results from the increased expression of Cxcr4 on tubular cells and macrophages and the accumulation of Cxcr4+ immune cells in the interstitium of the injured kidney.
Cxcr4 Antagonist AMD Blunts the UUO-Induced Fibrotic Response
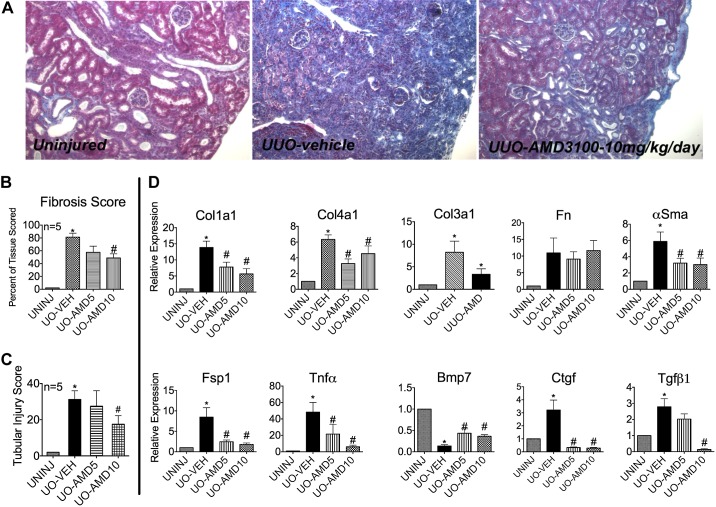
Having found that Cxcr4 expression is increased after UUO, we next tested whether this increase had any pathological consequence vis-à-vis fibrosis. We took the advantage of the Federal Drug Administration-approved pharmacological inhibitor of Cxcr4, AMD (18). Mice that underwent UUO were administered either vehicle (PBS) or AMD at 5 mg·kg−1·day−1 (AMD5 group) or 10 mg·kg−1·day−1 (AMD10 group) from the time of UUO until the day before euthanization. [Of note, mice can tolerate up to a 30 mg/kg dose of AMD (18).] Mice were euthanized on day 12 after UUO, and kidneys analyzed for matrix deposition (Masson's trichrome stain) and gene expression of classic indicators of fibrosis. Figure 2A shows representative Masson's trichrome staining from the respective study groups. Mice that had received 5 mg/kg AMD did not achieve a statistically significant decrease in fibrosis score, although they trended toward a lower score. In contrast, the AMD10 group had a significantly lower fibrosis score (∼45%) compared with the vehicle-treated group (Fig. 2, A and B). In addition, the AMD10 group had a lower tubular injury score, as shown by the bar graph in Fig. 2C. Tubular injury leading to tubular cell death has been shown to directly correlate with the progression of fibrosis (20).
Fig. 2.
The Cxcr4 antagonist AMD-3100 (AMD) blunts the UUO-induced fibrotic response. Mice underwent UUO for 12 days. Mice (n = 5 mice/group) received vehicle [Veh (PBS)], 5 mg/kg AMD (AMD5), or 10 mg/kg AMD (AMD10) via daily subcutaneous injections. A: representative Masson's trichrome staining of kidneys from the three groups (magnification: ×200). The AMD10 group had a significantly lower fibrosis score (B) and reduced tubular injury score (C). RT-PCR was performed to determine total mRNA levels of classic markers of fibrosis (D). The increase in transforming growth factor (TGF)-β1 mRNA was significantly blunted by 10 mg/kg AMD, which inversely correlated with bone morphogenetic protein 7 (Bmp7) mRNA expression. Col1a1, collagen type IA1; Col4a1, collagen type IVA1; Col3a1, collagen type IIIA1; Fn, fibronectin; α-SMA, α-smooth muscle actin; Fsp1, fibroblast-specific protein-1; Ctgf, connective tissue growth factor. n = 5. *P < 0.05.
Cxcr4 Antagonist Blunts the Increase in Classic Indicators of Fibrosis
In agreement with the Masson's trichrome stain data, significantly smaller increases in Col1a1, Col3a1, and collagen type IV (Col4)a1 mRNA were observed in mice administered AMD. No change in fibronectin was observed. AMD significantly blunted the increase in gene expression of Fsp1, α-SMA, TNF-α, connective tissue growth factor (CTGF), and TGF-β1. Interestingly, AMD significantly rescued Bmp7 expression that was suppressed by UUO. Bmp7 protects against fibrosis, likely by antagonizing TGF-β1-dependent fibrotic signals and by helping maintain a differentiation state of epithelial tubules (37, 57, 61, 74, 82, 85, 90).
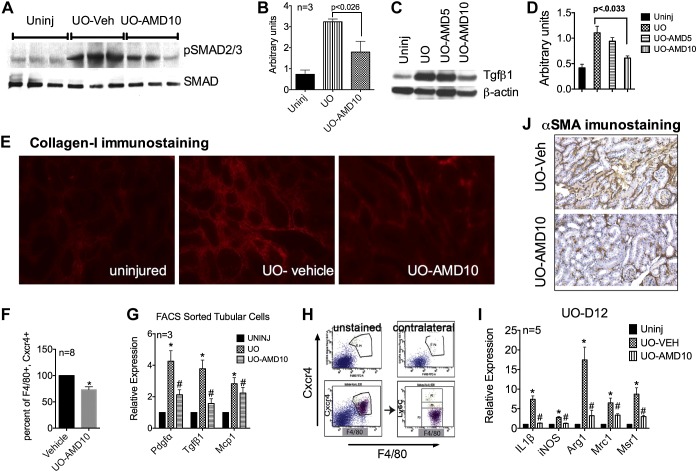
AMD Treatment Blunts Downstream Effects of TGF-β1 Signaling and Fibroblast Activation
Complimenting the decrease in TGF-β1 mRNA was the partial inhibition of TGF-β1-mediated downstream signaling, as demonstrated by the reduced phosphorylation of Smad2/3 shown in the representative immunoblot in Fig. 3A, where each lane represents an individual mouse. Quantification of the immunoblot is shown in Fig. 3B. Interestingly, a significant reduction in TGF-β1 protein content was observed only in AMD10 group, as shown in a representative immunoblot in Fig. 3C with the pooled data quantified in Fig. 3D. Similar to decreased gene expression, significantly less Col1 staining was observed in the AMD10 group, as shown in representative immunostained kidney sections from each group (Fig. 3E). Consistent with decreased Fsp1 and α-SMA mRNA content observed in AMD-treated groups, fewer α-SMA-positive cells were observed in the AMD10 group, shown in representative immunostained kidney sections from vehicle-treated and AMD10 groups (Fig. 3J).
Fig. 3.
AMD blunts UUO-induced TGF-β1 protein expression, signaling, collagen deposition, and macrophage activation. A–D: representative Western blot of phosphorylated (p)Smad2/3 from total kidney lysates of AMD-treated mice (n = 3; A), densitometry plot (B), representative Western blot showing total kidney TGF-β1 (C), and quantification from three separate blots (D). E: reduced Col1 staining was also observed. F: percent reduction in macrophage numbers in UUO kidneys (n = 8). G: AMD significantly blunted platelet-derived growth factor (PDGF)-α and TGF-β1 mRNA expression. MCP-1, monocyte chemotactic protein-1. Most of the Cxcr4+ macrophages are lymphocyte antigen (Ly)6Clo. H: representative FACS plot. I: mRNA expression of both pro- and anti-inflammatory macrophage activation was significantly blunted by AMD administration (n = 5). iNOS, inducible nitric oxide synthase; Arg1, arginase-1; Mrc1, mannose receptor C type 1; Msr1, macrophage scavenger receptor 1. J: representative image showing decreased fibroblast activation in tissue sections immunostained for α-SMA (magnification: ×200). *P < 0.05.
AMD Modestly Inhibits Macrophage Infiltration but Affects Their Activation
Despite an increase in Cxcr4 expression per macrophage (Fig. 1H) and a significant increase in the ligand Sdf-1α, the infiltration of F4/80+ Cxcr4+ myeloid cells after UUO was only partially blunted by the administration of 10 mg/kg AMD (∼26% decrease on day 6 after UUO, n = 8; Fig. 3F). This suggests that macrophage homing to the obstructed kidney is only partially dependent on Sdf-1-Cxcr4 signaling. However, this could be explained by only a modest decrease in monocyte chemotactic protein-1 mRNA of tubular cells from AMD-treated mice. In comparison, a significant decrease in platelet-derived growth factor (PDGF)-α and TGF-β1 mRNA was observed in FACS-sorted tubular cells from kidneys of AMD-treated mice (Fig. 3G).
Does Cxcr4 Expression on Macrophages Influence Their Anti- and/or Proinflammatory Characteristics?
Monocytes infiltrate the injured kidney as a F4/80+ Ly6Chigh population and later differentiate into “profibrotic” a F4/80+ Ly6Clo population (47). We analyzed the CD45+ F4/80+ Cxcr4+ cell population from day 9 UUO kidneys for Ly6C expression by FACS analysis and determined that >90% of the F4/80+ Cxcr4+ population was Ly6Clo (Fig. 3H), supporting our notion that the Cxcr4+ subset of macrophages may be profibrotic. To assess this, we analyzed whole kidney mRNA for pro- and anti-inflammatory markers of macrophages. After UUO, there was a severalfold increase in both proinflammatory genes (inducible nitric oxide synthase and IL-1β) as well as markers of alternative macrophage activation that are associated with fibrosis (argninase-1, Mrc1, and Msr1; Fig. 3I). However, AMD attenuated both anti- and proinflammatory markers of macrophage activation. This suggests that Cxcr4 signaling may not be specific to the profibrotic macrophage response. However, this requires further investigation.
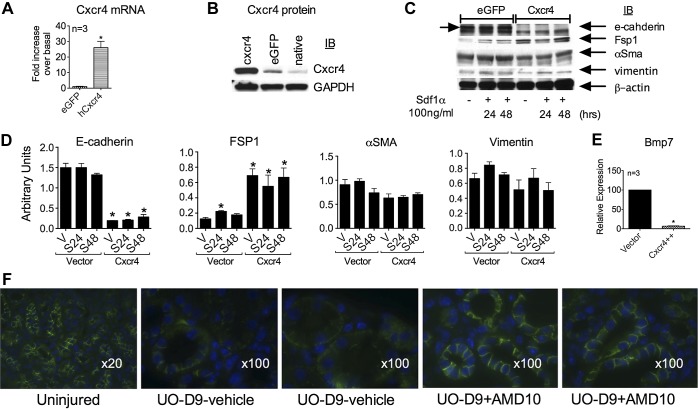
Overexpression of Cxcr4 in Proximal Tubule Cells Leads to Their Spontaneous Partial Dedifferentiation
To assess the impact of increased Cxcr4 expression on tubular epithelial cells, we overexpressed Cxcr4 in human proximal tubule cells (HKC cells). Overexpression was confirmed by quantitative RT-PCR and protein analysis of the cell lysates (Fig. 4, A and B). Overexpression of Cxcr4 in HKC cells led to the spontaneous loss of E-cadherin and an increase in Fsp1 expression without affecting a typical mesenchymal marker, vimentin (Fig. 4C; quantitated in Fig. 4D), suggesting that, indeed, a partial epithelial dedifferentiation can occur in the presence of excessive Cxcr4 content. This is in agreement with previously published reports (69, 75) demonstrating that higher Cxcr4 expression in tumor cells can lead to epithelia-to-mesenchymal-like changes. Similar to these in vitro findings, when UUO kidneys were immunostained for E-cadherin, a partial and mosaic pattern of loss of E-cadherin was observed in some of the tubules. This partial loss of E-cadherin was prevented by AMD treatment (Fig. 4F). Consistent with the in vivo results, overexpression of Cxcr4 in HKC cells caused a spontaneous loss of Bmp7 gene expression (Fig. 4E).
Fig. 4.
Overexpression of Cxcr4 leads to partial dedifferentiation of kidney epithelial tubular cells. Human proximal tubular cells were transduced with retroviral vector expressing enhanced green fluorescent protein (eGFP) or human (h)Cxcr4. A: mRNA overexpression was confirmed by quantitative RT-PCR. B: representative Western blot confirming protein overexpression. C: cells were serum starved for 4 h and stimulated with vehicle or 200 ng/ml Sdf-1α for 24 h (S24) or 48 h (S48). Equal amounts of protein lysate were immunoblotted for E-cadherin, Fsp1, α-SMA, and vimentin. D: representative blot and densitometry from four separate experiments. In vivo after UUO, there was a sporadic loss of E-cadherin, which was prevented by AMD administration. E: overexpression of Cxcr4 led to a spontaneous loss of Bmp7 mRNA. F: representative pictures of immunofluorescent staining for E-cadherin (green) in kidneys from uninjured mice and from two individual mice treated with either vehicle or 10 mg·kg−1·day−1 AMD for 9 days after UUO. Magnification: ×20 (uninjured group) and ×100 for treated groups.
Taken together, increased total Cxcr4 expression in UUO kidneys correlated with a worse fibrotic response that was blunted by Cxcr4 antagonist. These data also indicate that increased Cxcr4 expression in the injured kidney promotes tubular TGF-β1 while suppressing Bmp7 gene expression, suggesting a novel pathway of cross-talk between Cxcr4 and TGF-β signaling vis-à-vis fibrosis, which requires further studies.
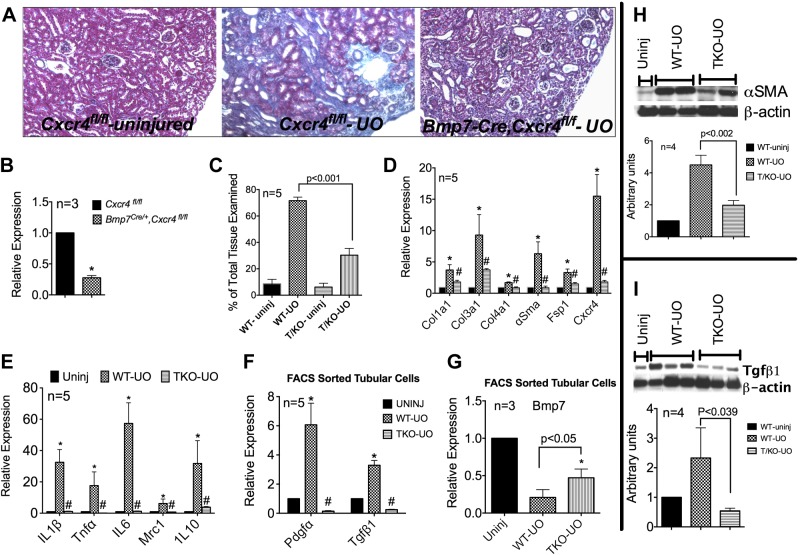
Nephron-Specific Ablation of Cxcr4 Partially Protects Against UUO-Induced Fibrosis
We generated kidney-specific Cxcr4 knockout mice by mating Bmp7Cre/+ mice with Cxcr4f/fl mice. Bmp7Cre/+ labels the entire developing nephron from the renal vesicle stage onward (59). Bmp7-Cre has been previously used for targeted deletion of Smad4 and c-Myc in the nephron (17, 59). Normal uninjured kidneys from 6- to 8-wk old Bmp7-Cre/+;Cxcr4f/fl mice [hereafter denoted as tubule knockout (TKO) mice] had an ∼80% reduction in total Cxcr4 mRNA content at baseline (Fig. 5B). These mice develop normally, and their kidneys appear normal on gross examination. When subjected to UUO, these mice were significantly protected from UUO-induced fibrosis. Representative Masson's trichrome staining is shown in Fig. 5A, and fibrosis scores averaged from five individual mice are shown in Fig. 5C. On average, ∼40% less fibrosis was observed in day 12 UUO TKO kidneys compared with Cxcr4fl/fl control kidneys. This correlated with significantly lower total kidney mRNA levels of Col1a1, Col3a1, Col4a1, α-SMA, Fsp1, and Cxcr4 (Fig. 5D). Complimenting the reduced fibrosis was a significantly muted expression of pro- and anti-inflammatory markers in UUO TKO kidneys, as indicated by lower mRNA levels of IL-1β, TNF-α, IL-6, and IL-10 (Fig. 5E). Interestingly, tubular cells FACS sorted from UUO TKO kidneys had significantly lower PDGF-α and TGF-β1 expression and partially protected Bmp7 expression. The decreased fibroblast activation, as suggested by reduced α-SMA gene expression, was confirmed by reduced α-SMA protein levels in UUO TKO kidneys, as shown in the representative immunoblot in Fig. 5H. The bar graph in Fig. 5 shows data pooled from 4 individual mice/group. Furthermore, a significant decrease in TGF-β1 protein expression was observed in UUO TKO kidneys, as shown by the representative Western blot in Fig. 5I. These data are consistent with the findings from AMD treatment in vivo and in vitro Cxcr4 overexpression experiments described above.
Fig. 5.
Nephron-specific deletion of Cxcr4 imparts partial protection against UUO-induced fibrosis. A: representative pictures of Masson's trichrome staining of kidneys from uninjured and day 12 UUO of Cxcr4fl/fl and Bmp7-Cre/+;Cxcr4fl/fl [tubule knockout (TKO)] mice. B: Whole kidney Cxcr4 mRNA expression in Cxcr4fl/fl and Bmp7,Cxcr4fl/fl mice. C: fibrosis scores averaged from five individual mice. D: whole kidney mRNA expression of markers of fibrosis and Cxcr4. E: macrophage activation. F and G: PDGF-α and TGF-β1 (F) and Bmp7 (G) mRNA expression in tubular cells FAC sorted from kidneys of uninjured, wild-type (WT), and TKO mice that underwent UUO. H and I: representative Western blots showing total kidney α-SMA (H) and TGF-β1 (I) expression in uninjured, UUO WT, and UUO TKO mice. Bar graphs show the quantification (relative to uninjured kidneys) of Western blots by densitometry pooled from four individual mice. n = 4. *P < 0.05 or as indicated.
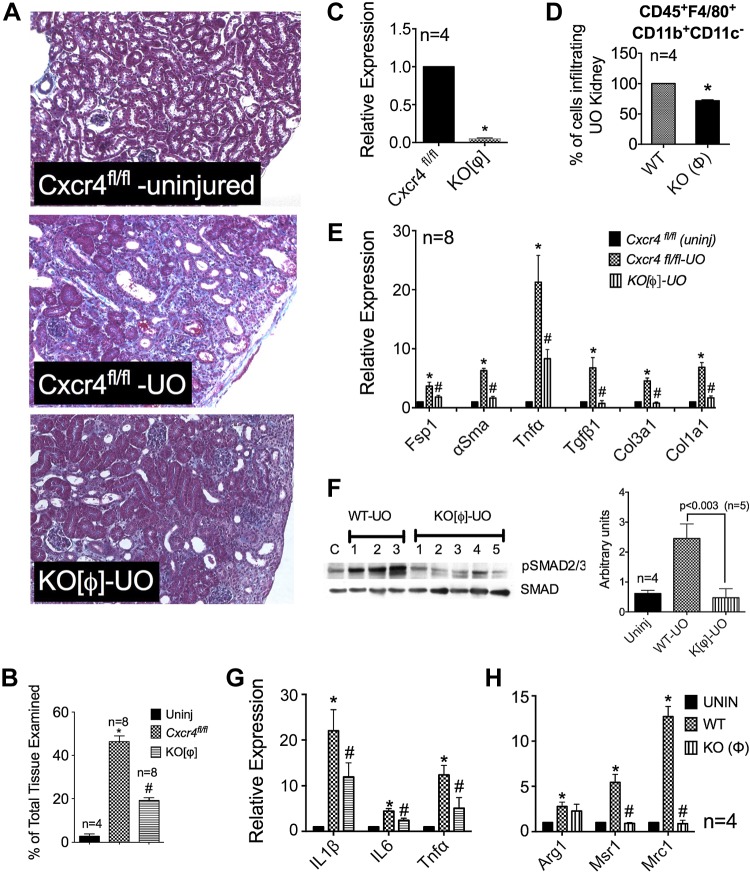
Selective Ablation of Cxcr4 in Macrophages Partially Protects Against Fibrosis
To specifically assess the role of macrophage Cxcr4 signaling in the UUO-induced fibrotic response, Cxcr4 was selectively ablated in macrophages by mating Cxcr4fl/fl mice with mice expressing the LysM-Cre allele. We achieved >90% reduction in Cxcr4 mRNA expression in macrophages derived from LysM-Cre;Cxcr4fl//fl mice (denoted as KO[Φ] mice), as determined by quantitative RT-PCR (Fig. 6C). These mice develop normally, and their kidneys appear normal on gross examination. KO[Φ] mice and their Cxcr4fl/fl littermates underwent UUO for 9 days. Figure 6A shows representative Masson's trichrome staining from the respective groups. Compared with Cxcr4fl/fl littermates, KO[Φ] mice developed significantly (∼50%) less fibrosis (Fig. 6B). mRNA analysis of whole kidneys revealed significantly decreased expression of TGF-β1 and markers of fibrosis, including Fsp1, α-SMA, TNF-α, Col1a1, and Col3a1 (Fig. 6E). This decrease in total TGF-β1 mRNA translated into a significant attenuation of its downstream signaling, as demonstrated by highly diminished pSmad2/3 phosphorylation in whole kidney lysates shown in the representative immunoblot in Fig. 6F (and quantified in the bar graph). Similar to the results seen with AMD treatment, FACS analysis of single cell suspensions from wild-type and KO[Φ] kidneys with UUO revealed that the lack of Cxcr4 led to ∼28% fewer macrophages infiltrating into UUO kidneys (n = 4; Fig. 6D). Knowing that Cxcr4 is important for homing of bone marrow-derived cells in the marrow, we examined whether KO[Φ] mice had increased numbers of circulating monocytes. Peripheral blood samples analyzed at baseline from wild-type or KO[Φ] mice did not show a statistically significant difference in the number of circulating monocytes between the two groups (data not shown).
Fig. 6.
Selective ablation of Cxcr4 in macrophages significantly blunts UUO-induced fibrosis. A: representative pictures of Masson's trichrome staining of kidneys from uninjured mice and day 9 UUO kidneys from Cxcr4fl/fl and LysM-Cre;Cxcr4fl//fl (KO[Φ]) mice. B: fibrosis score (in %, n = 8). C: a >90% decrease in Cxcr4 mRNA was achieved in Cxcr4-null macrophages. D: mice lacking Cxcr4 in macrophages showed significantly decreased fibrosis markers (n = 8). E and F: significant attenuation was observed in TGF-β1 signaling, as determined by pSmad2/3 immunoblot analysis (E) and the corresponding densitometry (n = 5; F). G: lack of Cxcr4 only modestly affected macrophage infiltration into UUO kidneys, as determined by FACS analysis of whole kidney lysates for CD45+ F4/80+ Cd11c− cells (n = 4). H: lack of Cxcr4 resulted in significant blunting of mRNA expression of profibrotic markers Msr1 and Mrc1, as determined in FACS-sorted macrophages from uninjured, UUO WT, and UUO KO[Φ] kidneys, and a modest reduction in IL-1β, IL-6, and TNF-α (n = 4; E). *P < 0.05 or as indicated.
Lack of Cxcr4 Suppresses Macrophage Activation
Macrophages (Cd45+ F4/80+ Cd11c−) were FACS sorted from uninjured and UUO kidneys of wild-type and KO[Φ] mice, and mRNA expression of anti- and proinflammatory markers were analyzed. Figure 6, G and H, shows data averaged from four individual mice. Similar to AMD treatment, loss of Cxcr4 on macrophages significantly prevented the induction of Msr1 and Mrc1 but only modestly affected arginase-1 (Fig. 6H). We also observed a modest decrease in proinflammatory markers, such as IL-1β, IL-6, and TNF-α. This suggests that Cxcr4 signaling in macrophages is required for both proinflammatory and profibrotic gene expression. Taken together, these data suggest that increased Cxcr4 expression in macrophages partly supports their trafficking to the injured kidney and their activation state. However, more detailed studies need to be carried out to further elucidate the role of Cxcr4 vis-à-vis alternative macrophage activation.
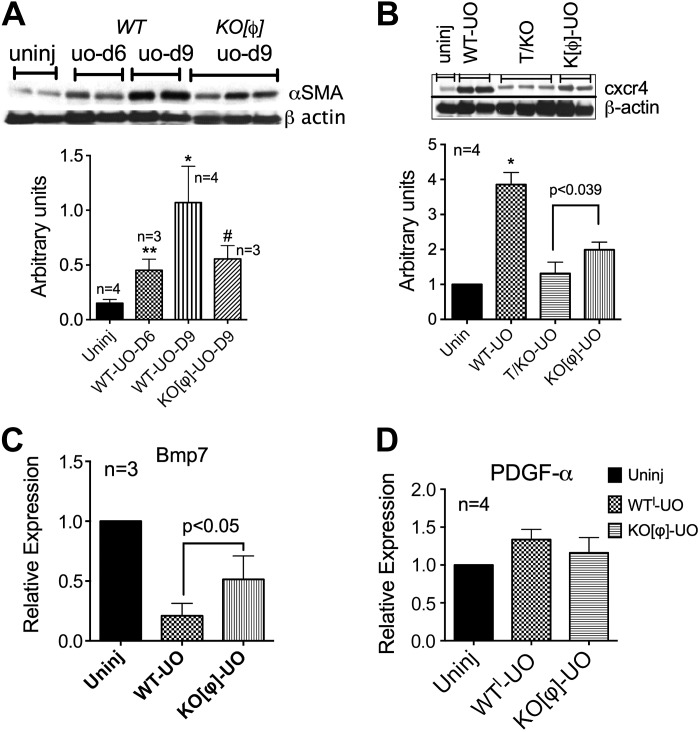
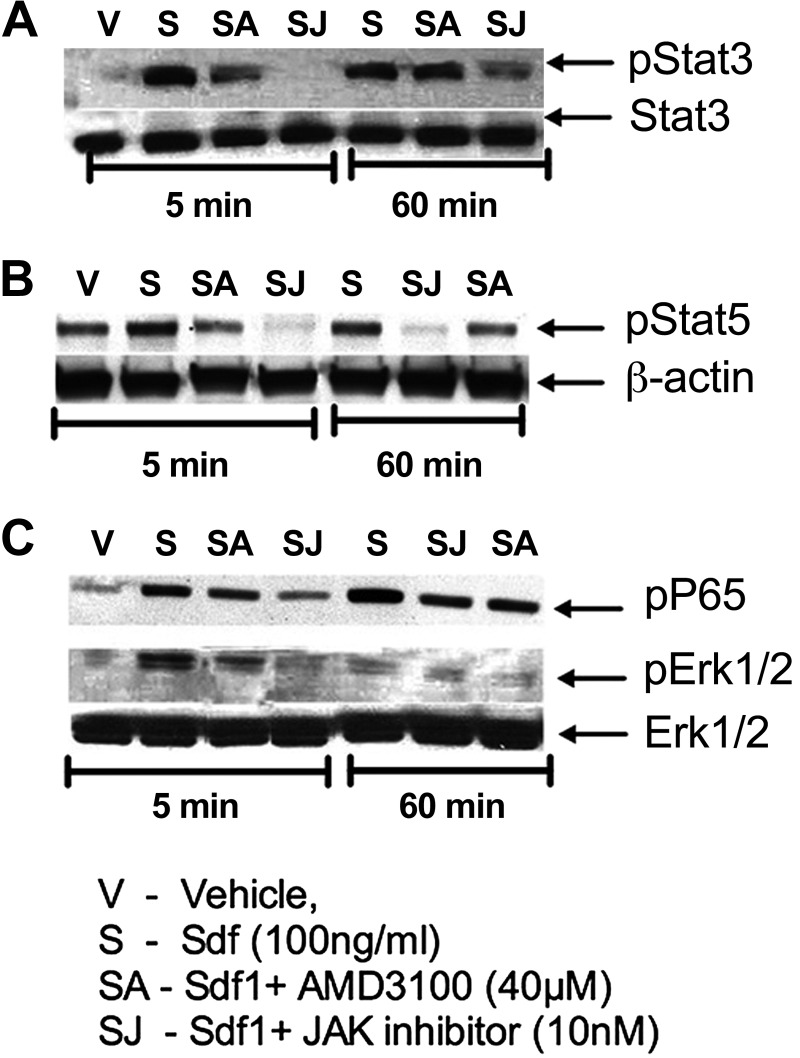
The total kidney decrease in TGF-β1 in UUO kidneys from KO[Φ] mice correlated with a significant decrease in α-SMA expression, suggesting blunted myofibroblast activation. Figure 7A shows a representative immunoblot and quantitative analysis in the bar graph. To determine the source of increased Cxcr4 observed in UUO kidneys in a definitive manner, we immunoblotted whole kidney lysates from UUO kidneys from TKO and KO[Φ] mice. As seen in the representative immunoblot and bar graph (Fig. 7B), the tubular contribution to increased Cxcr4 was greater than that of macrophages. However, it was apparent that even macrophage Cxcr4 signaling contributes toward overall Bmp7 expression, whereas it had no impact on PDGF-α mRNA expression (Fig. 7, C and D). Finally, we determined whether or not Cxcr4 signaling in macrophages activates known transcriptional signaling cascades that might influence their classic or alternate activation. Bone marrow-derived macrophages were stimulated with Sdf-1α, and lysates were immunoblotted for phosphorylated STAT3, STAT5, and p65. The representative immunoblots shown in Fig. 8 demonstrate that, indeed, multiple pathways were activated in macrophages by Cxcr4 stimulation. Further studies are required to determine which of these pathways might be relevant to the macrophage Cxcr4-mediated fibrotic response.
Fig. 7.
The tubular compartment contributes to most of the Cxcr4 increase after UUO, but conditional ablation of Cxcr4 in myeloid cells prevents loss of Bmp7. A and B: representative Western blots showing whole kidney α-SMA (A) and Cxcr4 (B) expression in uninjured and day 9 UUO kidneys from WT and KO[Φ] mice. Bar graphs plots show pooled densitometry values. C: whole kidney mRNA expression of Bmp7. D: PDGF-α mRNA expression in macrophages FACS sorted from kidneys of uninjured mice or WT and KO[Φ] mice that underwent UUO for 9 days. *P < 0.05 or as indicated.
Fig. 8.
Stimulation of Cxcr4 in bone marrow-derived macrophages (BMMs) activates multiple signaling pathways. BMMs were cultured as described in materials and methods. Cells were serum starved for 3 h and then stimulated with vehicle (PBS) or Sdf-1α (100 ng/ml) for 5 or 60 min in the presence or absence of AMD (40 μM) or 10 nM JAK inhibitor (JAK). Protein lysates (40 mg) were immunoblotted for pStat3 and Stat3 (A), pStat5 and β-actin (B), or pp65 (to assess NF-κB activation), pErk1/2, and total Erk1/2 (C). Experiments were repeated at least three times. Representative immunoblots are shown.
Taken together, we demonstrate that pathological Cxcr4 expression can contribute to kidney fibrosis via multiple effectors. Its significance in a disease process remains to be verified in an appropriate model, such as that of diabetic nephropathy.
DISCUSSION
Chemokine receptor Cxcr4 is historically known for its role in neural and immune system development as well as trafficking/homing of the bone marrow-derived immune and precursor cells (8). Cxcr4 is ubiquitously expressed. Overexpression of Cxcr4 has been linked to increased tumor progression by promoting dedifferentiation of epithelia (2, 10, 12, 32). Besides basal transcriptional regulation by nuclear respiratory factor-1 (58), Cxcr4 can be induced by a number of signaling molecules. These include cytokines IL-2, IL-4, IL-10, and TGF-β1 and by growth factors such as basic FGF, VEGF, and EGF (26, 38, 62, 80). On the other hand, inflammatory cytokines, such as IL-1β, interferon-γ, and TNF-α, all can attenuate Cxcr4 expression (28, 31, 60). Most of these molecules are highly upregulated in an injured kidney and can affect Cxcr4 expression in a cell-specific and temporal fashion. For example, hypoxia, which occurs in an injured kidney after small vessel rarefaction, induces Cxcr4 gene expression in various cell types, such as monocytes, monocyte-derived macrophages, endothelial cells, and cancer cells (63). Hyperexpression of Cxcr4 in multiple models of lupus nephritis positively correlates with the progression of lupus (79). More recently, Ding and coworkers (19) reported that chronic injury to the liver (by repeated carbon tetrachloride injection and bile duct ligation) augmented Cxcr4 expression in liver sinusoidal endothelial cells, and this enforces a source of the profibrotic response. Increased Cxcr4 expression is also seen in tubular segments after renal ischemia-reperfusion injury (73, 91). Intense Cxcr4 staining can be observed in tubular segments of human biopsies of IgA nephropathy, minimal-change nephrotic syndrome, focal segmental glomerulosclerosis, membranoproliferative glomerulonephritis, chronic pyelonephritis, and acute tubular necrosis (50), but its significance has not been well studied. Cxcr4 is also highly upregulated on monocytes, neutrophils, B cell subsets, and plasma cells in multiple murine models of lupus with active nephritis (79). Thus, there is an association of increased Cxcr4 expression with a chronic inflammatory or injury state.
In the present study, we demonstrated a gradual increase in Cxcr4 expression in tubular and CD45+ cells after UUO. Antagonizing Cxcr4 in vivo by AMD attenuated the fibrotic response to UUO, thereby demonstrating a pathological consequence to the sustained increase in Cxcr4 expression in the kidney. Similar to what is known in certain tumor cells, overexpression of Cxcr4 in proximal tubular cells led to a spontaneous loss of E-cadherin. However, unlike tumors, there was no gain of mesenchymal markers. In vivo, loss of E-cadherin in tubules of UUO kidney had a mosaic pattern. Although this may suggest a partial dedifferentiation due to a chronic increase of Cxcr4 expression in tubular cells, since we could not costain for E-cadherin and Cxcr4, such a conclusion would be speculative. However, the fact that tubular cells from UUO TKO kidneys expressed less PDGF-α, TGF-β1, etc. supports the idea that increased Cxcr4 can lead to a partial dedifferentiated state of tubular cells in vivo, leading to the secretion of cytokines (etc.) that could further affect the interstitial homeostasis. This was also supported by the loss of Bmp7 expression in the presence of increased Cxcr4, both in vitro and in vivo.
The lower α-SMA and TGF-β1 expression in UUO kidneys of AMD-treated and TKO groups suggests a significant role of tubular Cxcr4 signaling in myofibroblast activation, i.e., reduced proliferation and/or muted fibroblast activation. Activated myofibroblasts are a major source of matrix deposition in the fibrotic kidney as well as in other organs (4, 11, 45, 84, 88). Both AMD and selective ablation of Cxcr4 in tubules led to reduced collagen deposition. Consistent with the reduced tubular injury in UUO kidneys of AMD-treated mice, there was a reduced inflammatory response, as judged by mRNA levels of both proinflammatory (IL-1β, TNF-α, and IL-6) and anti-inflammatory (Mrc1 and IL-10) markers. Since IL-10 is known to induce Cxcr4 expression (38), reduced IL-10 could further contribute by reducing Cxcr4 expression on IL-10 receptor-positive cells. These results are further supported by a recent study demonstrating a protective effect of AMD on tubular injury, after ischemia-reperfusion, that eventually led to a reduced long-term fibrotic response as well (91).
The cumulative increase in macrophage Cxcr4 expression may reflect the changing milieu within the obstructed kidney such that there is increased hypoxia, TGF-β1, IL-10, and IL-4, all of which can augment Cxcr4 expression (8). The partial inhibition of macrophage infiltration (into a UUO kidney) by AMD is likely due the presence of another major macrophage chemoattractant, monocyte chemotactic protein-1, which is upregulated after UUO and only modestly decreased by Cxcr4 antagonist. Selective ablation of Cxcr4 in macrophages too led to only a modest ∼23% decrease in the number of macrophages homing to the obstructed kidney while still providing a significant protection against fibrosis. This raises the possibility that such a modest decrease in trafficking of macrophages may be enough to achieve significant beneficial effects. Alternatively, this suggests an additional role of Cxcr4 in the macrophage-mediated fibrotic response.
Profibrotic (M2) macrophages derived from human peripheral blood show ∼16-fold greater Cxcr4 expression (54), but its significance is not known. Historically, at least in rodents, these alternately activated (M2) macrophages have been associated with tissue repair, such as repair after an acute kidney injury (35, 46). However, a prolonged presence of these prorepair macrophages can promote fibrosis (21, 22, 33, 42, 77). Cxcr4 signaling activates multiple signaling pathways that are dependent on Gi proteins, such as PI3K, eventually leading to regulation of gene transcription, cell adhesion, and cell migration (7, 8). However, G protein-independent signaling via activation of JAK/STAT too has been proposed (78). Consistent with these findings, we determined that stimulation of Cxcr4 in naïve bone marrow-derived macrophages activated multiple STAT and NF-κB pathways (Fig. 8). These signaling pathways play important roles in macrophage activation toward pro- and anti-inflammatory states (30, 44, 65, 71). We had hypothesized that increased Cxcr4 expression on macrophages will promote a profibrotic phenotype. However, our data demonstrate that Cxcr4 signaling can modulate macrophage activation and drive both pro- and anti-inflammatory phenotypes. However, since we did not FACS sort macrophages based on their level of Cxcr4 expression, it is conceivable that in our experiments, we had a mixed population of high and low Cxcr4-expressing macrophages. Thus, we cannot conclude that increased Cxcr4 on macrophages favors their profibrotic phenotype, as was hypothesized. However, identifying the specific pathway promoting fibrosis is beyond the scope of the present study and requires further detailed studies. Two recent studies (52, 64) have demonstrated antifibrotic effects of Cxcr4 antagonist in bleomycin- and radiation-induced lung fibrosis. The two studies listed fibrocytes and bone marrow-derived mesenchymal cells, respectively, as the potential effectors carrying fibrotic signals in the lung, thus further highlighting that Cxcr4 can modulate fibrotic effects via multiple effectors.
In summary, this study demonstrates that after injury, Cxcr4 expression is upregulated in multiple cell types, which influences their biology and thereby contributes to the progression of fibrosis. In the tubular cell compartment, it promotes a partially dedifferentiated phenotype promoting the secretion of cytokines, etc., and in macrophages it impacts their trafficking and activation state. Activation of Cxcr4 in bone marrow-derived macrophages activates multiple STAT and NF-κB pathways that are known to regulate macrophage activation. Which specific signaling pathways might be involved in Cxcr4-mediated divergent effects remain to be determined. The fact that Cxcr4 signaling can regulate Bmp7, PDGF-α, and TGF-β1 expression is a novel finding and uncovers a new pathway. Identifying more downstream targets of Cxcr4 signaling in multiple effector cell types opens new avenues for therapeutic intervention in fibrosis. One also needs to keep in mind that Cxcr4 signaling may contribute to regenerative pathways too and may thus be an important protective signal, as has been recently demonstrated (14). Thus, an important next step would be to demonstrate the significance of this pathway in the setting of CKD, such as diabetic nephropathy.
GRANTS
This work was supported by the George M. O'Brien Kidney Center at Yale (National Institute of Diabetes and Digestive and Kidney Diseases Grant P30-DK-079310) and a grant-in-aid from the American Heart Association (to A. Karihaloo).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.Y., Y.L., and U.C. performed experiments; G.W.M. and A.K. analyzed data; A.K. conception and design of research; A.K. interpreted results of experiments; A.K. drafted manuscript; A.K. edited and revised manuscript; A.K. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors sincerely thank Dr. Lloyd Cantley for helpful discussions during the course of this study.
REFERENCES
- 1.Appaiah H, Bhat-Nakshatri P, Mehta R, Thorat M, Badve S, Nakshatri H. ITF2 is a target of CXCR4 in MDA-MB-231 breast cancer cells and is associated with reduced survival in estrogen receptor-negative breast cancer. Cancer Biol Ther 10: 600–614, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbieri F, Bajetto A, Stumm R, Pattarozzi A, Porcile C, Zona G, Dorcaratto A, Ravetti JL, Minuto F, Spaziante R, Schettini G, Ferone D, Florio T. Overexpression of stromal cell-derived factor 1 and its receptor CXCR4 induces autocrine/paracrine cell proliferation in human pituitary adenomas. Clin Cancer Res 14: 5022–5032, 2008. [DOI] [PubMed] [Google Scholar]
- 3.Bettink SI, Werner C, Chen CH, Muller P, Schirmer SH, Walenta KL, Bohm M, Laufs U, Friedrich EB. Integrin-linked kinase is a central mediator in angiotensin II type 1- and chemokine receptor CXCR4 signaling in myocardial hypertrophy. Biochem Biophys Res Commun 397: 208–213, 2010. [DOI] [PubMed] [Google Scholar]
- 4.Borges FT, Melo SA, Ozdemir BC, Kato N, Revuelta I, Miller CA, Gattone VH 2nd, LeBleu VS, Kalluri R. TGF-β1-containing exosomes from injured epithelial cells activate fibroblasts to initiate tissue regenerative responses and fibrosis. J Am Soc Nephrol 24: 385–392, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brenner S, Whiting-Theobald N, Kawai T, Linton GF, Rudikoff AG, Choi U, Ryser MF, Murphy PM, Sechler JM, Malech HL. CXCR4-transgene expression significantly improves marrow engraftment of cultured hematopoietic stem cells. Stem Cells 22: 1128–1133, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Buckley CD, Amft N, Bradfield PF, Pilling D, Ross E, Arenzana-Seisdedos F, Amara A, Curnow SJ, Lord JM, Scheel-Toellner D, Salmon M. Persistent induction of the chemokine receptor CXCR4 by TGF-β1 on synovial T cells contributes to their accumulation within the rheumatoid synovium. J Immunol 165: 3423–3429, 2000. [DOI] [PubMed] [Google Scholar]
- 7.Busillo JM, Armando S, Sengupta R, Meucci O, Bouvier M, Benovic JL. Site-specific phosphorylation of CXCR4 is dynamically regulated by multiple kinases and results in differential modulation of CXCR4 signaling. J Biol Chem 285: 7805–7817, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Busillo JM, Benovic JL. Regulation of CXCR4 signaling. Biochim Biophys Acta 1768: 952–963, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cabioglu N, Sahin A, Doucet M, Yavuz E, Igci A, OY E, Aktas E, Bilgic S, Kiran B, Deniz G, Price JE. Chemokine receptor CXCR4 expression in breast cancer as a potential predictive marker of isolated tumor cells in bone marrow. Clin Exp Metastasis 22: 39–46, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Campanholle G, Ligresti G, Gharib SA, Duffield JS. Cellular mechanisms of tissue fibrosis. 3. Novel mechanisms of kidney fibrosis. Am J Physiol Cell Physiol 304: C591–C603, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campioni D, Lo Monaco A, Lanza F, Moretti S, Ferrari L, Fotinidi M, La Corte R, Cuneo A, Trotta F. CXCR4 pos circulating progenitor cells coexpressing monocytic and endothelial markers correlating with fibrotic clinical features are present in the peripheral blood of patients affected by systemic sclerosis. Haematologica 93: 1233–1237, 2008. [DOI] [PubMed] [Google Scholar]
- 13.Chen D, Chen Z, Park C, Centrella M, McCarthy T, Chen L, Al-Omari A, Moeckel GW. Aldosterone stimulates fibronectin synthesis in renal fibroblasts through mineralocorticoid receptor-dependent and independent mechanisms. Gene 531: 23–30, 2013. [DOI] [PubMed] [Google Scholar]
- 14.Chen LH, Advani SL, Thai K, Kabir MG, Sood MM, Gibson IW, Yuen DA, Connelly KA, Marsden PA, Kelly DJ, Gilbert RE, Advani A. SDF-1/CXCR4 signaling preserves microvascular integrity and renal function in chronic kidney disease. PLos One 9: e92227, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen S, Tuttle DL, Oshier JT, Knot HJ, Streit WJ, Goodenow MM, Harrison JK. Transforming growth factor-β1 increases CXCR4 expression, stromal-derived factor-1α-stimulated signalling and human immunodeficiency virus-1 entry in human monocyte-derived macrophages. Immunology 114: 565–574, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chinni SR, Sivalogan S, Dong Z, Filho JC, Deng X, Bonfil RD, Cher ML. CXCL12/CXCR4 signaling activates Akt-1 and MMP-9 expression in prostate cancer cells: the role of bone microenvironment-associated CXCL12. Prostate 66: 32–48, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Couillard M, Trudel M. C-myc as a modulator of renal stem/progenitor cell population. Dev Dyn 238: 405–414, 2009. [DOI] [PubMed] [Google Scholar]
- 18.De Clercq E. The AMD3100 story: the path to the discovery of a stem cell mobilizer (Mozobil). Biochem Pharmacol 77: 1655–1664, 2009. [DOI] [PubMed] [Google Scholar]
- 19.Ding BS, Cao Z, Lis R, Nolan DJ, Guo P, Simons M, Penfold ME, Shido K, Rabbany SY, Rafii S. Divergent angiocrine signals from vascular niche balance liver regeneration and fibrosis. Nature 505: 97–102, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Docherty NG, O'Sullivan OE, Healy DA, Fitzpatrick JM, Watson RW. Evidence that inhibition of tubular cell apoptosis protects against renal damage and development of fibrosis following ureteric obstruction. Am J Physiol Renal Physiol 290: F4–F13, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Duffield JS. Macrophages and immunologic inflammation of the kidney. Semin Nephrol 30: 234–254, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duffield JS. Macrophages in kidney repair and regeneration. J Am Soc Nephrol 22: 199–201, 2011. [DOI] [PubMed] [Google Scholar]
- 23.Duffield JS, Tipping PG, Kipari T, Cailhier JF, Clay S, Lang R, Bonventre JV, Hughes J. Conditional ablation of macrophages halts progression of crescentic glomerulonephritis. Am J Pathol 167: 1207–1219, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eardley KS, Kubal C, Zehnder D, Quinkler M, Lepenies J, Savage CO, Howie AJ, Kaur K, Cooper MS, Adu D, Cockwell P. The role of capillary density, macrophage infiltration and interstitial scarring in the pathogenesis of human chronic kidney disease. Kidney Int 74: 495–504, 2008. [DOI] [PubMed] [Google Scholar]
- 25.Eardley KS, Zehnder D, Quinkler M, Lepenies J, Bates RL, Savage CO, Howie AJ, Adu D, Cockwell P. The relationship between albuminuria, MCP-1/CCL2, and interstitial macrophages in chronic kidney disease. Kidney Int 69: 1189–1197, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Feil C, Augustin HG. Endothelial cells differentially express functional CXC-chemokine receptor-4 (CXCR-4/fusin) under the control of autocrine activity and exogenous cytokines. Biochem Biophys Res Commun 247: 38–45, 1998. [DOI] [PubMed] [Google Scholar]
- 27.Flier SN, Tanjore H, Kokkotou EG, Sugimoto H, Zeisberg M, Kalluri R. Identification of epithelial to mesenchymal transition as a novel source of fibroblasts in intestinal fibrosis. J Biol Chem 285: 20202–20212, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gupta SK, Lysko PG, Pillarisetti K, Ohlstein E, Stadel JM. Chemokine receptors in human endothelial cells. Functional expression of CXCR4 and its transcriptional regulation by inflammatory cytokines. J Biol Chem 273: 4282–4287, 1998. [DOI] [PubMed] [Google Scholar]
- 30.Hagemann T, Biswas SK, Lawrence T, Sica A, Lewis CE. Regulation of macrophage function in tumors: the multifaceted role of NF-κB. Blood 113: 3139–3146, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han Y, Wang J, He T, Ransohoff RM. TNF-α down-regulates CXCR4 expression in primary murine astrocytes. Brain Res 888: 1–10, 2001. [DOI] [PubMed] [Google Scholar]
- 32.Hashimoto N, Jin H, Liu T, Chensue SW, Phan SH. Bone marrow-derived progenitor cells in pulmonary fibrosis. J Clin Invest 113: 243–252, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Henderson NC, Mackinnon AC, Farnworth SL, Kipari T, Haslett C, Iredale JP, Liu FT, Hughes J, Sethi T. Galectin-3 expression and secretion links macrophages to the promotion of renal fibrosis. Am J Pathol 172: 288–298, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang YC, Hsiao YC, Chen YJ, Wei YY, Lai TH, Tang CH. Stromal cell-derived factor-1 enhances motility and integrin up-regulation through CXCR4, ERK and NF-κB-dependent pathway in human lung cancer cells. Biochem Pharmacol 74: 1702–1712, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Huen SC, Cantley LG. Macrophage-mediated injury and repair after ischemic kidney injury. Pediatr Nephrol 30: 199–209, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ishikawa T, Nakashiro K, Klosek SK, Goda H, Hara S, Uchida D, Hamakawa H. Hypoxia enhances CXCR4 expression by activating HIF-1 in oral squamous cell carcinoma. Oncol Rep 21: 707–712, 2009. [PubMed] [Google Scholar]
- 37.Jena N, Martin-Seisdedos C, McCue P, Croce CM. BMP7 null mutation in mice: developmental defects in skeleton, kidney, and eye. Exp Cell Res 230: 28–37, 1997. [DOI] [PubMed] [Google Scholar]
- 38.Jourdan P, Vendrell JP, Huguet MF, Segondy M, Bousquet J, Pene J, Yssel H. Cytokines and cell surface molecules independently induce CXCR4 expression on CD4+ CCR7+ human memory T cells. J Immunol 165: 716–724, 2000. [DOI] [PubMed] [Google Scholar]
- 39.Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest 112: 1776–1784, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karihaloo A, Koraishy F, Huen SC, Lee Y, Merrick D, Caplan MJ, Somlo S, Cantley LG. Macrophages promote cyst growth in polycystic kidney disease. J Am Soc Nephrol 22: 1809–1814, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karihaloo A, Koraishy F, Huen SC, Lee Y, Merrick D, Caplan MJ, Somlo S, Cantley LG. Macrophages promote cyst growth in polycystic kidney disease. J Am Soc Nephrol 22: 1809–1814, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ko GJ, Boo CS, Jo SK, Cho WY, Kim HK. Macrophages contribute to the development of renal fibrosis following ischaemia/reperfusion-induced acute kidney injury. Nephrol Dial Transplant 23: 842–852, 2008. [DOI] [PubMed] [Google Scholar]
- 43.Lagane B, Chow KY, Balabanian K, Levoye A, Harriague J, Planchenault T, Baleux F, Gunera-Saad N, Arenzana-Seisdedos F, Bachelerie F. CXCR4 dimerization and β-arrestin-mediated signaling account for the enhanced chemotaxis to CXCL12 in WHIM syndrome. Blood 112: 34–44, 2008. [DOI] [PubMed] [Google Scholar]
- 44.Lawrence T, Natoli G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat Rev Immunol 11: 750–761, 2011. [DOI] [PubMed] [Google Scholar]
- 45.LeBleu VS, Taduri G, O'Connell J, Teng Y, Cooke VG, Woda C, Sugimoto H, Kalluri R. Origin and function of myofibroblasts in kidney fibrosis. Nat Med 19: 1047–1053, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee S, Huen S, Nishio H, Nishio S, Lee HK, Choi BS, Ruhrberg C, Cantley LG. Distinct macrophage phenotypes contribute to kidney injury and repair. J Am Soc Nephrol 22: 317–326, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin SL, Castano AP, Nowlin BT, Lupher ML Jr, Duffield JS. Bone marrow Ly6Chigh monocytes are selectively recruited to injured kidney and differentiate into functionally distinct populations. J Immunol 183: 6733–6743, 2009. [DOI] [PubMed] [Google Scholar]
- 48.Lin SL, Kisseleva T, Brenner DA, Duffield JS. Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am J Pathol 173: 1617–1627, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Loetscher P, Moser B, Baggiolini M. Chemokines and their receptors in lymphocyte traffic and HIV infection. Adv Immunol 74: 127–180, 2000. [DOI] [PubMed] [Google Scholar]
- 50.Lotan D, Sheinberg N, Kopolovic J, Dekel B. Expression of SDF-1/CXCR4 in injured human kidneys. Pediatr Nephrol 23: 71–77, 2008. [DOI] [PubMed] [Google Scholar]
- 51.Luo Y, Cai J, Xue H, Mattson MP, Rao MS. SDF1α/CXCR4 signaling stimulates β-catenin transcriptional activity in rat neural progenitors. Neurosci Lett 398: 291–295, 2006. [DOI] [PubMed] [Google Scholar]
- 52.Makino H, Aono Y, Azuma M, Kishi M, Yokota Y, Kinoshita K, Takezaki A, Kishi J, Kawano H, Ogawa H, Uehara H, Izumi K, Sone S, Nishioka Y. Antifibrotic effects of CXCR4 antagonist in bleomycin-induced pulmonary fibrosis in mice. J Med Invest 60: 127–137, 2013. [DOI] [PubMed] [Google Scholar]
- 53.Martinez FO. Analysis of gene expression and gene silencing in human macrophages. Curr Protoc Immunol Chapter 14: unit 14.28-23, 2012. [DOI] [PubMed] [Google Scholar]
- 54.Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol 177: 7303–7311, 2006. [DOI] [PubMed] [Google Scholar]
- 55.Mason S, Hader C, Marlier A, Moeckel G, Cantley LG. Met activation is required for early cytoprotection after ischemic kidney injury. J Am Soc Nephrol 25: 329–337, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mehrad B, Burdick MD, Strieter RM. Fibrocyte CXCR4 regulation as a therapeutic target in pulmonary fibrosis. Int J Biochem Cell Biol 41: 1708–1718, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mitu G, Hirschberg R. Bone morphogenetic protein-7 (BMP7) in chronic kidney disease. Front Biosci 13: 4726–4739, 2008. [DOI] [PubMed] [Google Scholar]
- 58.Moriuchi M, Moriuchi H, Turner W, Fauci AS. Cloning and analysis of the promoter region of CXCR4, a coreceptor for HIV-1 entry. J Immunol 159: 4322–4329, 1997. [PubMed] [Google Scholar]
- 59.Oxburgh L, Chu GC, Michael SK, Robertson EJ. TGFβ superfamily signals are required for morphogenesis of the kidney mesenchyme progenitor population. Development 131: 4593–4605, 2004. [DOI] [PubMed] [Google Scholar]
- 60.Phillips RJ, Mestas J, Gharaee-Kermani M, Burdick MD, Sica A, Belperio JA, Keane MP, Strieter RM. Epidermal growth factor and hypoxia-induced expression of CXC chemokine receptor 4 on non-small cell lung cancer cells is regulated by the phosphatidylinositol 3-kinase/PTEN/AKT/mammalian target of rapamycin signaling pathway and activation of hypoxia inducible factor-1α. J Biol Chem 280: 22473–22481, 2005. [DOI] [PubMed] [Google Scholar]
- 61.Piscione TD, Phan T, Rosenblum ND. BMP7 controls collecting tubule cell proliferation and apoptosis via Smad1-dependent and -independent pathways. Am J Physiol Renal Physiol 280: F19–F33, 2001. [DOI] [PubMed] [Google Scholar]
- 62.Salcedo R, Wasserman K, Young HA, Grimm MC, Howard OM, Anver MR, Kleinman HK, Murphy WJ, Oppenheim JJ. Vascular endothelial growth factor and basic fibroblast growth factor induce expression of CXCR4 on human endothelial cells: in vivo neovascularization induced by stromal-derived factor-1α. Am J Pathol 154: 1125–1135, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schioppa T, Uranchimeg B, Saccani A, Biswas SK, Doni A, Rapisarda A, Bernasconi S, Saccani S, Nebuloni M, Vago L, Mantovani A, Melillo G, Sica A. Regulation of the chemokine receptor CXCR4 by hypoxia. J Exp Med 198: 1391–1402, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shu HK, Yoon Y, Hong S, Xu K, Gao H, Hao C, Torres-Gonzalez E, Nayra C, Rojas M, Shim H. Inhibition of the CXCL12/CXCR4-axis as preventive therapy for radiation-induced pulmonary fibrosis. PLos One 8: e79768, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 122: 787–795, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Strutz F, Zeisberg M. Renal fibroblasts and myofibroblasts in chronic kidney disease. J Am Soc Nephrol 17: 2992–2998, 2006. [DOI] [PubMed] [Google Scholar]
- 67.Tachibana K, Hirota S, Iizasa H, Yoshida H, Kawabata K, Kataoka Y, Kitamura Y, Matsushima K, Yoshida N, Nishikawa S, Kishimoto T, Nagasawa T. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature 393: 591–594, 1998. [DOI] [PubMed] [Google Scholar]
- 68.Takabatake Y, Sugiyama T, Kohara H, Matsusaka T, Kurihara H, Koni PA, Nagasawa Y, Hamano T, Matsui I, Kawada N, Imai E, Nagasawa T, Rakugi H, Isaka Y. The CXCL12 (SDF-1)/CXCR4 axis is essential for the development of renal vasculature. J Am Soc Nephrol 20: 1714–1723, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Taki M, Higashikawa K, Yoneda S, Ono S, Shigeishi H, Nagayama M, Kamata N. Up-regulation of stromal cell-derived factor-1α and its receptor CXCR4 expression accompanied with epithelial-mesenchymal transition in human oral squamous cell carcinoma. Oncol Rep 19: 993–998, 2008. [PubMed] [Google Scholar]
- 70.Tang CH, Chuang JY, Fong YC, Maa MC, Way TD, Hung CH. Bone-derived SDF-1 stimulates IL-6 release via CXCR4, ERK and NF-κB pathways and promotes osteoclastogenesis in human oral cancer cells. Carcinogenesis 29: 1483–1492, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Timmer AM, Nizet V. IKKβ/NF-κB and the miscreant macrophage. J Exp Med 205: 1255–1259, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Togel F, Isaac J, Hu Z, Weiss K, Westenfelder C. Renal SDF-1 signals mobilization and homing of CXCR4-positive cells to the kidney after ischemic injury. Kidney Int 67: 1772–1784, 2005. [DOI] [PubMed] [Google Scholar]
- 73.Togel F, Isaac J, Hu Z, Weiss K, Westenfelder C. Renal SDF-1 signals mobilization and homing of CXCR4-positive cells to the kidney after ischemic injury. Kidney Int 67: 1772–1784, 2005. [DOI] [PubMed] [Google Scholar]
- 74.Tomita M, Asada M, Asada N, Nakamura J, Oguchi A, Higashi AY, Endo S, Robertson E, Kimura T, Kita T, Economides AN, Kreidberg J, Yanagita M. Bmp7 maintains undifferentiated kidney progenitor population and determines nephron numbers at birth. PLos One 8: e73554, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ueda Y, Neel NF, Schutyser E, Raman D, Richmond A. Deletion of the COOH-terminal domain of CXC chemokine receptor 4 leads to the down-regulation of cell-to-cell contact, enhanced motility and proliferation in breast carcinoma cells. Cancer Res 66: 5665–5675, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ueland J, Yuan A, Marlier A, Gallagher AR, Karihaloo A. A novel role for the chemokine receptor Cxcr4 in kidney morphogenesis: an in vitro study. Dev Dyn 238: 1083–1091, 2009. [DOI] [PubMed] [Google Scholar]
- 77.Vernon MA, Mylonas KJ, Hughes J. Macrophages and renal fibrosis. Semin Nephrol 30: 302–317, 2010. [DOI] [PubMed] [Google Scholar]
- 78.Vila-Coro AJ, Rodriguez-Frade JM, Martin De Ana A, Moreno-Ortiz MC, Martinez AC, Mellado M. The chemokine SDF-1α triggers CXCR4 receptor dimerization and activates the JAK/STAT pathway. FASEB J 13: 1699–1710, 1999. [PubMed] [Google Scholar]
- 79.Wang A, Fairhurst AM, Tus K, Subramanian S, Liu Y, Lin F, Igarashi P, Zhou XJ, Batteux F, Wong D, Wakeland EK, Mohan C. CXCR4/CXCL12 hyperexpression plays a pivotal role in the pathogenesis of lupus. J Immunol 182: 4448–4458, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang J, Guan E, Roderiquez G, Calvert V, Alvarez R, Norcross MA. Role of tyrosine phosphorylation in ligand-independent sequestration of CXCR4 in human primary monocytes-macrophages. J Biol Chem 276: 49236–49243, 2001. [DOI] [PubMed] [Google Scholar]
- 81.Wang Q, Diao X, Sun J, Chen Z. Regulation of VEGF, MMP-9 and metastasis by CXCR4 in a prostate cancer cell line. Cell Biol Int 35: 897–904, 2011. [DOI] [PubMed] [Google Scholar]
- 82.Wang S, Hirschberg R. BMP7 antagonizes TGF-β-dependent fibrogenesis in mesangial cells. Am J Physiol Renal Physiol 284: F1006–F1013, 2003. [DOI] [PubMed] [Google Scholar]
- 83.Wang Y, Cui L, Gonsiorek W, Min SH, Anilkumar G, Rosenblum S, Kozlowski J, Lundell D, Fine JS, Grant EP. CCR2 and CXCR4 regulate peripheral blood monocyte pharmacodynamics and link to efficacy in experimental autoimmune encephalomyelitis. J Inflamm 6: 32, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wu CF, Chiang WC, Lai CF, Chang FC, Chen YT, Chou YH, Wu TH, Linn GR, Ling H, Wu KD, Tsai TJ, Chen YM, Duffield JS, Lin SL. Transforming growth factor β-1 stimulates profibrotic epithelial signaling to activate pericyte-myofibroblast transition in obstructive kidney fibrosis. Am J Pathol 182: 118–131, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yasmin N, Bauer T, Modak M, Wagner K, Schuster C, Koffel R, Seyerl M, Stockl J, Elbe-Burger A, Graf D, Strobl H. Identification of bone morphogenetic protein 7 (BMP7) as an instructive factor for human epidermal Langerhans cell differentiation. J Exp Med 210: 2597–2610, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yonemoto S, Machiguchi T, Nomura K, Minakata T, Nanno M, Yoshida H. Correlations of tissue macrophages and cytoskeletal protein expression with renal fibrosis in patients with diabetes mellitus. Clin Exp Nephrol 10: 186–192, 2006. [DOI] [PubMed] [Google Scholar]
- 87.Zeisberg EM, Potenta SE, Sugimoto H, Zeisberg M, Kalluri R. Fibroblasts in kidney fibrosis emerge via endothelial-to-mesenchymal transition. J Am Soc Nephrol 19: 2282–2287, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zeisberg M, Kalluri R. Cellular mechanisms of tissue fibrosis. 1. Common and organ-specific mechanisms associated with tissue fibrosis. Am J Physiol Cell Physiol 304: C216–C225, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zeisberg M, Kalluri R. Fibroblasts emerge via epithelial-mesenchymal transition in chronic kidney fibrosis. Front Biosci 13: 6991–6998, 2008. [DOI] [PubMed] [Google Scholar]
- 90.Zeisberg M, Kalluri R. Reversal of experimental renal fibrosis by BMP7 provides insights into novel therapeutic strategies for chronic kidney disease. Pediatr Nephrol 23: 1395–1398, 2008. [DOI] [PubMed] [Google Scholar]
- 91.Zuk A, Gershenovich M, Ivanova Y, MacFarland RT, Fricker SP, Ledbetter S. CXCR4 antagonism as a therapeutic approach to prevent acute kidney injury. Am J Physiol Renal Physiol 307: F783–F797, 2014. [DOI] [PubMed] [Google Scholar]