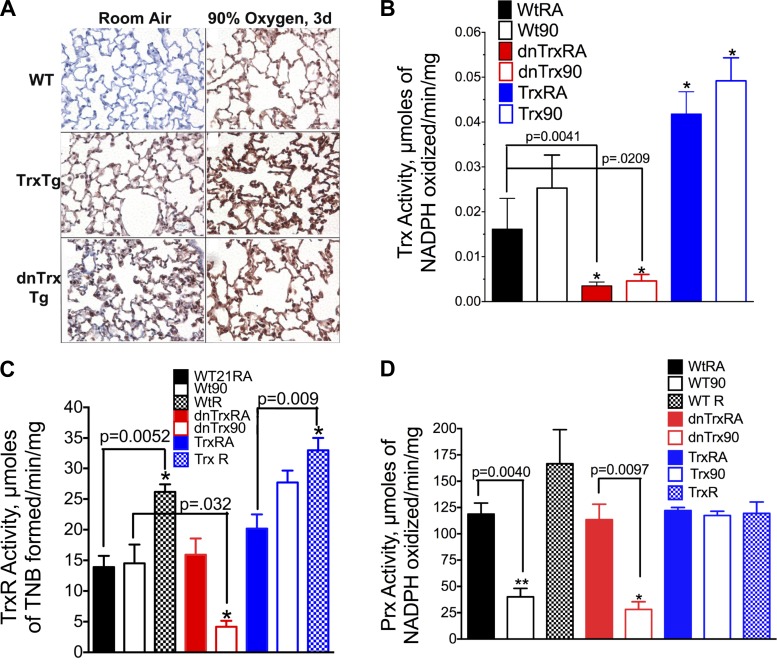
Fig. 3.
A: increased expression of Trx in lungs of WT, Trx-Tg, and dnTrx-Tg mice in hyperoxia. Lungs of mice were inflation fixed with 10% neutral formalin, and paraffin sections were processed for immunohistochemistry. Primary anti-Trx antibody used here was obtained from Santa Cruz Biotechnology. This antibody is reactive to both mouse and human protein: Top left: WT mouse lung in room air; top right: lungs of WT mice in hyperoxia. Middle left; lungs of Trx-Tg mice in room air; Middle right: Lungs of Trx-Tg mice in hyperoxia. Bottom left: Lung of dnTrx mice in room air; bottom right: Lungs of dnTrx mice in hyperoxia. Brown stain represents Trx or dnTrx protein in the lung sections of mice. B: activity of Trx is increased in the lungs of Trx-Tg mice in normoxia as well as hyperoxia but decreased in the dnTrx-Tg mice: lung tissue was homogenized and the Trx activity assay was performed as described in materials and methods. The activity was expressed as μmol NADPH oxidized·min−1·mg lung protein−1 at 25°C. The means were compared using Student's t-test. C: expression of TrxR decreases in the lungs of dnTrx-Tg mice in hyperoxia but increases during recovery in WT/NT and Trx-Tg mice. Mice were exposed to 90% oxygen for 4 days followed by 3 days of recovery in room air. TrxR activity was determined by formation thio-2-nitro benzene (TNB) that was monitored at 412 nm as described in materials and methods. *Significantly increased or decreased compared with WT, Trx-Tg, or dnTrx-Tg mice. D: activity of peroxiredoxins (Prx) decreased in WT or dnTrx-Tg mice in hyperoxia, but remains unchanged in Trx-Tg mice. Mice were exposed to 90% oxygen for 4 days followed by 3 days of recovery in room air. Prx activity was determined using H2O2 as a substrate in presence of Trx-TrxR system as described in the methods. *,**Significantly lower than WT, Trx-Tg, or dnTrx-Tg mice in normoxia.