Abstract
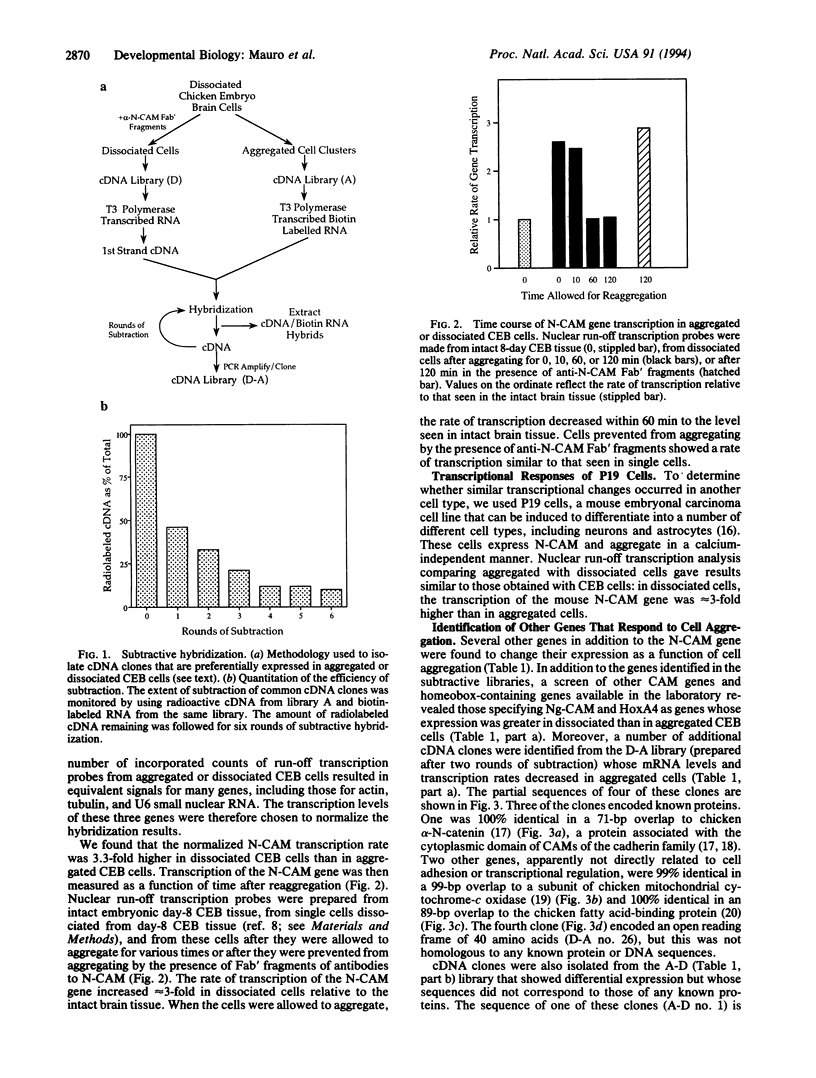
To determine whether changes in gene expression occur in embryonic cells as a consequence of changes in cellular aggregation, chicken embryo brain (CEB) cells isolated from 8-day embryos were allowed to aggregate or prevented from aggregating by treatment with anti-neural cell adhesion molecule (N-CAM) Fab' fragments. A subtractive hybridization cloning strategy was employed to identify genes that might show different levels of expression in the two populations of cells. In addition, the transcription rates of a number of genes specifying CAMs and transcription factors were directly estimated by using nuclear run-off transcription assays. The transcription rates of several genes, including those encoding N-CAM, Ng-CAM, alpha-N-catenin, HoxA4 (Hox1.4), a fatty acid-binding protein, and a subunit of the mitochondrially encoded cytochrome-c oxidase enzyme decreased upon CEB cell aggregation. The transcription rates of several previously unidentified genes either increased or decreased upon aggregation, while the transcription of other genes remained unchanged. The transcription rate of the N-CAM gene was 3.3-fold higher in dissociated than in aggregated CEB cells. This rate of transcription also increased when the brain tissue was dissociated into single cells and the increased rate was maintained by keeping the cells dissociated in the presence of Fab' fragments of antibodies to N-CAM. Decreased transcription rates of the N-CAM gene were also observed upon aggregation of P19 cells, a mouse embryonal carcinoma cell line. Primary chicken embryo liver cells, which aggregate primarily by calcium-dependent adhesion mechanisms, did not show changes in the N-CAM gene or in the other genes whose transcription rates changed in CEB cells and P19 cells. These observations suggest that the types of genes regulated by cell aggregation include those for CAMs themselves as well as for transcription factors that may control the expression of CAMs and other molecules significant for morphogenesis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bastian H., Gruss P. A murine even-skipped homologue, Evx 1, is expressed during early embryogenesis and neurogenesis in a biphasic manner. EMBO J. 1990 Jun;9(6):1839–1852. doi: 10.1002/j.1460-2075.1990.tb08309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begemann M., Tan S. S., Cunningham B. A., Edelman G. M. Expression of chicken liver cell adhesion molecule fusion genes in transgenic mice. Proc Natl Acad Sci U S A. 1990 Nov;87(22):9042–9046. doi: 10.1073/pnas.87.22.9042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behringer R. R., Crotty D. A., Tennyson V. M., Brinster R. L., Palmiter R. D., Wolgemuth D. J. Sequences 5' of the homeobox of the Hox-1.4 gene direct tissue-specific expression of lacZ during mouse development. Development. 1993 Mar;117(3):823–833. doi: 10.1242/dev.117.3.823. [DOI] [PubMed] [Google Scholar]
- Brackenbury R., Rutishauser U., Edelman G. M. Distinct calcium-independent and calcium-dependent adhesion systems of chicken embryo cells. Proc Natl Acad Sci U S A. 1981 Jan;78(1):387–391. doi: 10.1073/pnas.78.1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackenbury R., Thiery J. P., Rutishauser U., Edelman G. M. Adhesion among neural cells of the chick embryo. I. An immunological assay for molecules involved in cell-cell binding. J Biol Chem. 1977 Oct 10;252(19):6835–6840. [PubMed] [Google Scholar]
- Desjardins P., Morais R. Sequence and gene organization of the chicken mitochondrial genome. A novel gene order in higher vertebrates. J Mol Biol. 1990 Apr 20;212(4):599–634. doi: 10.1016/0022-2836(90)90225-B. [DOI] [PubMed] [Google Scholar]
- Doherty P., Ashton S. V., Moore S. E., Walsh F. S. Morphoregulatory activities of NCAM and N-cadherin can be accounted for by G protein-dependent activation of L- and N-type neuronal Ca2+ channels. Cell. 1991 Oct 4;67(1):21–33. doi: 10.1016/0092-8674(91)90569-k. [DOI] [PubMed] [Google Scholar]
- Edelman G. M. A golden age for adhesion. Cell Adhes Commun. 1993 May;1(1):1–7. doi: 10.3109/15419069309095677. [DOI] [PubMed] [Google Scholar]
- Edelman G. M. Morphoregulation. Dev Dyn. 1992 Jan;193(1):2–10. doi: 10.1002/aja.1001930103. [DOI] [PubMed] [Google Scholar]
- Godbout R. Identification and characterization of transcripts present at elevated levels in the undifferentiated chick retina. Exp Eye Res. 1993 Jan;56(1):95–106. doi: 10.1006/exer.1993.1014. [DOI] [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Hirano S., Kimoto N., Shimoyama Y., Hirohashi S., Takeichi M. Identification of a neural alpha-catenin as a key regulator of cadherin function and multicellular organization. Cell. 1992 Jul 24;70(2):293–301. doi: 10.1016/0092-8674(92)90103-j. [DOI] [PubMed] [Google Scholar]
- Howard L. A., Ortlepp S. A. Construction of cloning/sequencing vectors with an alternative polylinker. Biotechniques. 1989 Oct;7(9):940–942. [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Jones F. S., Chalepakis G., Gruss P., Edelman G. M. Activation of the cytotactin promoter by the homeobox-containing gene Evx-1. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2091–2095. doi: 10.1073/pnas.89.6.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones F. S., Holst B. D., Minowa O., De Robertis E. M., Edelman G. M. Binding and transcriptional activation of the promoter for the neural cell adhesion molecule by HoxC6 (Hox-3.3). Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6557–6561. doi: 10.1073/pnas.90.14.6557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones F. S., Prediger E. A., Bittner D. A., De Robertis E. M., Edelman G. M. Cell adhesion molecules as targets for Hox genes: neural cell adhesion molecule promoter activity is modulated by cotransfection with Hox-2.5 and -2.4. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2086–2090. doi: 10.1073/pnas.89.6.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessel M., Gruss P. Murine developmental control genes. Science. 1990 Jul 27;249(4967):374–379. doi: 10.1126/science.1974085. [DOI] [PubMed] [Google Scholar]
- Klar A., Baldassare M., Jessell T. M. F-spondin: a gene expressed at high levels in the floor plate encodes a secreted protein that promotes neural cell adhesion and neurite extension. Cell. 1992 Apr 3;69(1):95–110. doi: 10.1016/0092-8674(92)90121-r. [DOI] [PubMed] [Google Scholar]
- Mauro V. P., Krushel L. A., Cunningham B. A., Edelman G. M. Homophilic and heterophilic binding activities of Nr-CAM, a nervous system cell adhesion molecule. J Cell Biol. 1992 Oct;119(1):191–202. doi: 10.1083/jcb.119.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa M., Baribault H., Kemler R. The cytoplasmic domain of the cell adhesion molecule uvomorulin associates with three independent proteins structurally related in different species. EMBO J. 1989 Jun;8(6):1711–1717. doi: 10.1002/j.1460-2075.1989.tb03563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuch U., Lohse M. J., Schachner M. Neural cell adhesion molecules influence second messenger systems. Neuron. 1989 Jul;3(1):13–20. doi: 10.1016/0896-6273(89)90111-6. [DOI] [PubMed] [Google Scholar]
- Sive H. L., St John T. A simple subtractive hybridization technique employing photoactivatable biotin and phenol extraction. Nucleic Acids Res. 1988 Nov 25;16(22):10937–10937. doi: 10.1093/nar/16.22.10937. [DOI] [PMC free article] [PubMed] [Google Scholar]


