Abstract
Trypanosoma cruzi is the causative agent of Chagas' disease, a chronic illness affecting 10 million people around the world. The complement system plays an important role in fighting microbial infections. The recognition molecules of the lectin pathway of complement activation, mannose-binding lectin (MBL), ficolins, and CL-11, bind to specific carbohydrates on pathogens, triggering complement activation through MBL-associated serine protease-2 (MASP-2). Previous in vitro work showed that human MBL and ficolins contribute to T. cruzi lysis. However, MBL-deficient mice are only moderately compromised in their defense against the parasite, as they may still activate the lectin pathway through ficolins and CL-11. Here, we assessed MASP-2-deficient mice, the only presently available mouse line with total lectin pathway deficiency, for a phenotype in T. cruzi infection. Total absence of lectin pathway functional activity did not confer higher susceptibility to T. cruzi infection, suggesting that it plays a minor role in the immune response against this parasite.
Chagas' disease (American Trypanosomiasis) is an endemic and chronic illness1 that affects around 10 million people in Latin America2 and ∼400,000 people in North America, Europe, and Asia.3 Its causative agent, the flagellated protozoan Trypanosoma cruzi, is transmitted through blood-feeding triatomine bugs, oral and transplacental routes, organ transplants, and blood transfusions.4 Infection with the parasite results in acute parasitemia, followed by a long asymptomatic indeterminate phase. Decades after the initial infection, ∼30% of the individuals progress to a chronic phase, characterized by severe cardiomyopathy or pathological enlargement of the digestive tract.5 Even though immunity plays a fundamental role in the parasite/mammalian host equilibrium,6 T. cruzi has developed effective strategies to escape the host immune response, including the immune defense mediated by the complement system.7–10
Three distinct pathways initiate the complement cascade: the classical, the alternative, and the lectin pathways.11 Lectin pathway activation permits an instant innate immune response to pathogens in naive hosts.12 Its activation is initiated through the recognition of pathogen-associated carbohydrate or acetylation patterns, present on microbial surfaces, by either the lectin domains of mannose-binding lectin (MBL) or collectin-11 (CL-11), or the fibrinogen-like domains of ficolins, which, upon binding to their respective pathogen-associated patterns, may initiate complement activation through conversion of the three different MBL-associated serine proteases (MASP) described, MASP-1, -2, and -3, into their enzymatically active form.13–17 Several studies suggest that MASP-2 alone is sufficient to trigger lectin complement activation, because mice deficient in MASP-1 and -3 maintain residual lectin pathway activity,18–21 which is completely absent in mice deficient of MASP-2.22
The role of the lectin pathway during the mammalian host immune defense against several pathogenic agents has been well established in both humans and mice, as shown by the association between increased infection susceptibility to parasitic diseases and genetic deficiencies in key components of the lectin pathway, such as MBL and ficolins.23–27 In addition, compromised MASP-2 functional activity caused by a polymorphism in the coding sequence of the human masp2 gene, leading to the loss of a critical calcium binding site, has been associated with autoimmune manifestations and predispositions to frequent infections.28,29
Previous in vitro reports have shown that human serum MBL and ficolins can bind to glycosylated molecules on the surface of T. cruzi metacyclic trypomastigotes, resulting in MASP-2-dependent complement activation.30 Conversely, a significant decrease in the deposition of complement activation products C3b and C4b on the parasite surface, and compromised complement-mediated parasite lysis, have been described in either MBL, L-ficolin, or H-ficolin-depleted human sera,30,31 suggesting that lectin pathway recognition molecules not only bind to the parasite surface, but also promote complement activation by the lectin pathway.
Additionally, an in vivo study using a mouse model of experimental trypanosomiasis compared the severity of the parasitic disease between MBL-null mice, which are deficient in both of the murine MBL genes, MBL-A and MBL-C,32 with that of their matched wild-type (WT) controls.33 The results revealed that MBL-null mice presented with a moderately increased parasitemia load and cardiac pathology,33 indicating that MBL regulates host resistance and cardiac inflammation following T. cruzi infection. Nevertheless, the MBL-null mouse strain fails to provide a reliable model for total lectin pathway deficiency, because they can still activate the lectin pathway through ficolin A, ficolin B, and/or CL-11.34,35 In addition, the MBL-null phenotype also includes the loss of MBL-mediated biological activities, which are independent of complement activation.32
In this study, we report the assessment of a MASP-2-deficient mouse line as the only presently available mouse model of total lectin pathway deficiency (which retains fully functional activity of both the classical and the alternative complement pathways)22 in experimental T. cruzi infection. The phenotype of MASP-2-deficient mice exclusively defines the loss of MASP-2-mediated complement activation, whereas all complement independent functions of the serum resident lectin pathway recognition components are retained.22
We evaluated the phenotype of MASP-2-deficient mice bred on a C57BL/6 background,22 a mouse strain with a genetic background that is highly susceptible to T. cruzi infection.36 All experimental procedures involving animals, which were maintained under internationally accepted guidelines, were approved by our Institutional Bioethics Committee (Faculty of Medicine, University of Chile). Genotyping using heteroduplex polymerase chain reaction (PCR) was performed to screen for MASP-2-deficient (MASP-2-/-) and WT alleles (MASP-2+/+) in the progeny of heterozygous intercrosses to generate littermate controls, as described previously22 (data not shown).
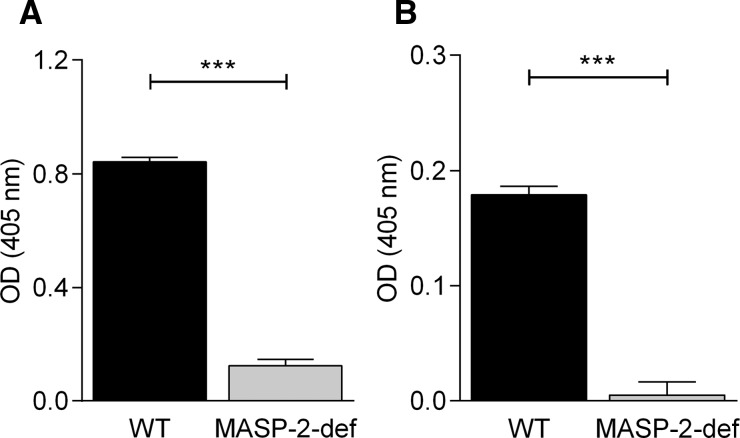
The lack of lectin pathway-dependent complement activation in MASP-2-deficient mice was confirmed in vitro using C4 cleavage assays on both, mannan or lipoteichoic acid-coated enzyme-linked immunosorbent assay (ELISA) plates, as described previously.22 As shown in Figure 1, lectin pathway-dependent C4 turnover was only detectable in WT mice sera, whereas sera of homozygous MASP-2-deficient mice presented with a total absence of C4 cleavage.
Figure 1.
The lectin pathway is not functional in MBL-associated serine protease (MASP)-2-deficient mice. Enzyme-linked immunosorbent assay (ELISA) plates were coated with (A) 1 μg/well of mannan from S. cerevisiae (Sigma-Aldrich, USA) or (B) 5 μg/well of lipoteichoic acid (LTA) (Sigma-Aldrich) in 100 μL carbonate buffer. Control wells received buffer alone. Residual protein binding sites were blocked using 1% bovine serum albumin (BSA) in Tris-buffered saline (TBS), followed by several washes. Sera from 15 wild-type (WT) and 6 MASP-2-deficient (MASP-2-def) mice, diluted in high salt binding buffer (20 mM Tris-HCl, 10 mM CaCl2, 1 M NaCl, pH 7.4), were added to plates coated with mannan (serum dilution 1/160) or LTA (serum dilution 1/20). C4 (Complement Technology Inc., USA) (0.05 μg/well in 50 μL barbital buffered saline) was added, and C4b deposition was detected with goat anti-human C4 IgG (Calbiochem), followed by incubation with horseradish peroxidase-conjugated rabbit anti-goat IgG (Calbiochem). Enzyme activities were assessed, at 405 nm, after addition of 2,2′-Azinobis [3-ethylbenzothiazoline-6-sulfonic acid]-diammonium salt (ABTS) with hydrogen peroxide. Background values of unspecific reactivity with BSA were subtracted from OD values obtained upon reactivity with carbohydrates. Results are from three independent experiments in triplicate wells. Differences between groups were determined by the Student's t test using GraphPad Prism Software v6.01 (USA). Mean values ± SE are shown (***P < 0.001).
We subsequently challenged 10 female and 5 male WT littermates and 2 female and 4 male MASP-2-deficient mice with an intraperitoneal inoculation of 100 T. cruzi trypomastigotes of the virulent Tulahuén strain.36 All mice were 12 weeks of age when challenged with parasites. For each mice group (MASP-2-deficient and WT controls), optical microscopy was carried out every 2 (or 3) days during 28 days after parasite inoculation to determine the number of circulating parasites in fresh blood obtained from tip cuts of mice tails, following standard protocols.37 The survival of infected mice was monitored on a daily basis. Mice that did not succumb to infection were euthanized 82 days after parasite inoculation, following internationally accepted procedures.
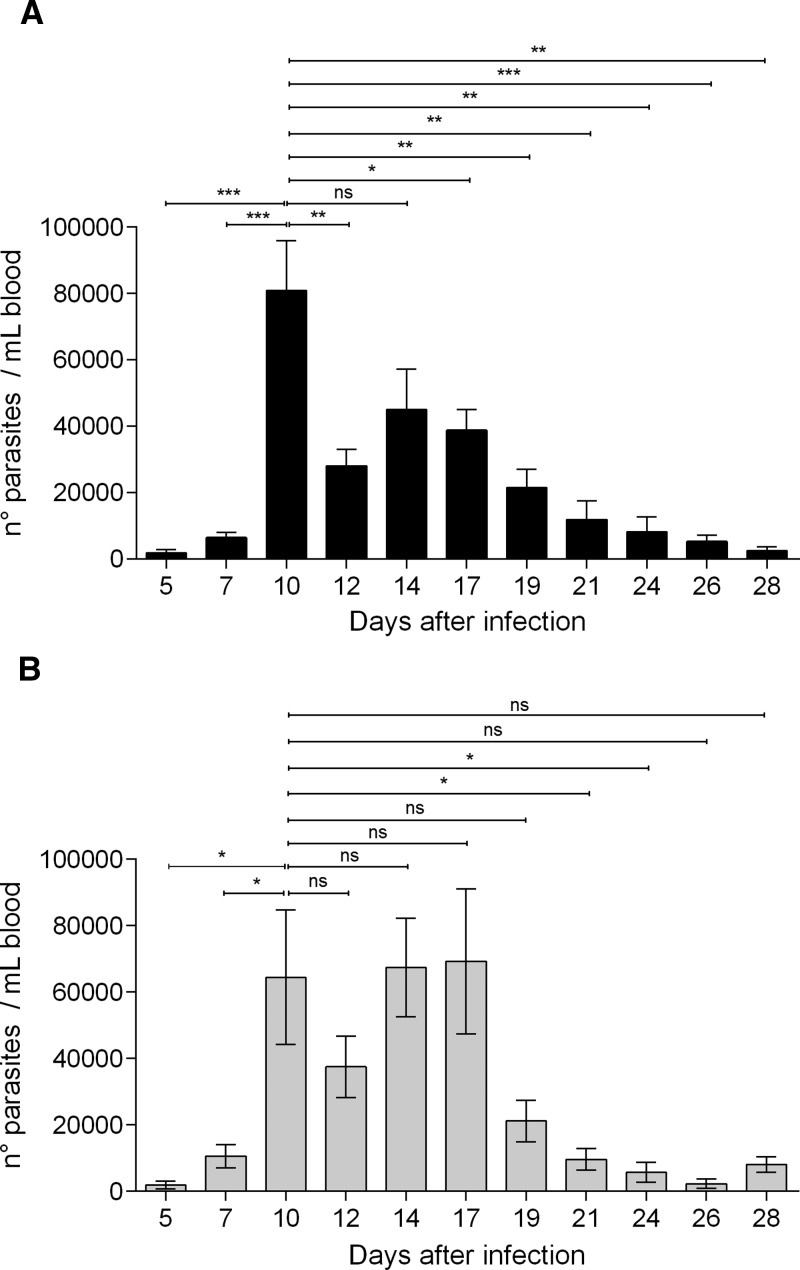
The WT mice reached the highest parasitemia load at Day 10 post-infection (p.i.) (Figure 2A). In these mice, the circulating parasite burden decreased significantly 2 days later (i.e., Day 12 p.i.), followed by a constant and steady decrease until the end of the observation time (Day 28 p.i.) (Figure 2A). However, MASP-2-deficient mice presented a high parasitemia load on Days 10, 14, and 17 p.i. (Figure 2B). Such high and sustained parasitemia burden was observed until Day 19 p.i., which decreased significantly only 2 days later (Day 21 p.i.) (Figure 2B).
Figure 2.
MBL-associated serine protease (MASP)-2-deficiency promotes sustained high circulating parasite load. Fifteen wild-type (WT) (A) and 6 MASP-2-def (B) 12-week-old mice received an intraperitoneal inoculation of 100 bloodstream T. cruzi trypomastigotes (Tulahuén strain). The number of parasites per mL of blood was estimated every 2 or 3 days, by microscopical counting, during 28 days after parasite inoculation. Differences between parasitemia values within each mice group were determined by the Student's t test. The results represent the mean ± SE values. Ns = non-significant; ***P < 0.001; **P < 0.01; *P < 0.05 in relation to parasitemia load on Day 10 p.i. for both mice groups. All data were analyzed with GraphPad Prism software.
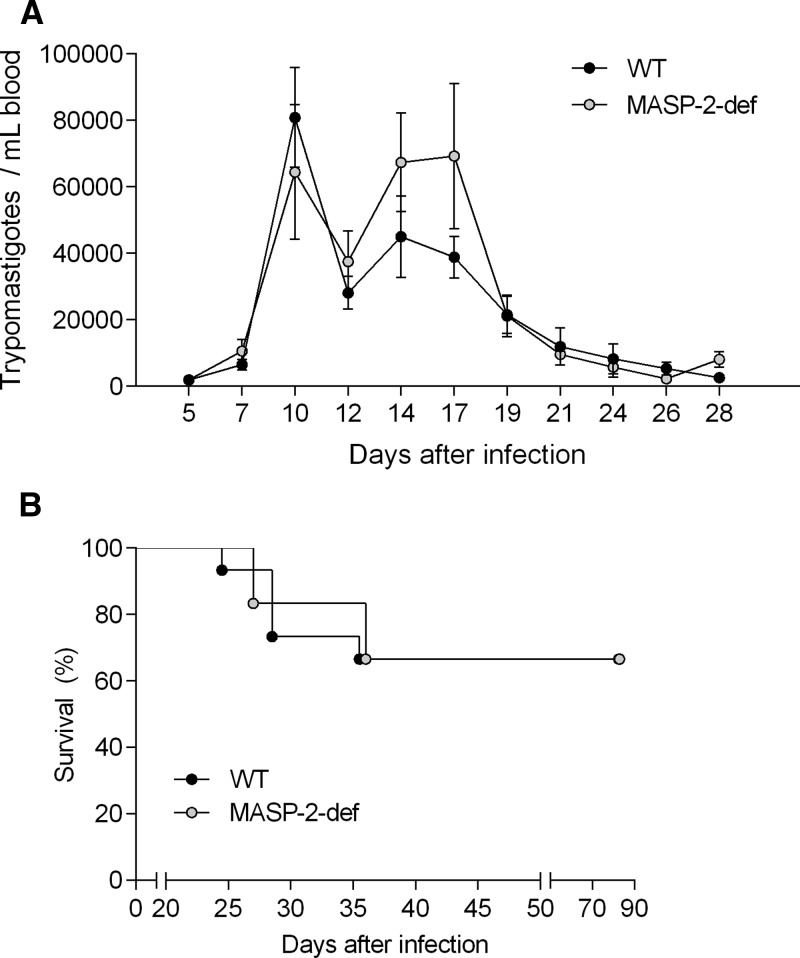
Surprisingly, even though WT mice were able to begin peripheral parasite clearing earlier than MASP-2-deficient mice (Figure 2), no significant difference in parasite load could be detected between infected MASP-2-deficient and WT mice at any time point of the acute experimental infection (Figure 3A).
Figure 3.
MBL-associated serine protease (MASP)-2-deficiency does not increase susceptibility to T. cruzi infection. (A) Parasitemia curves of 15 wild-type (WT) and 6 MASP-2-def mice infected with T. cruzi trypomastigotes (Figure 2) were compared between mice groups by two-way analysis of variance followed by Sidak's analysis for multiple comparisons. The results represent the mean ± SE values. (B) Kaplan-Meier curves for survival rates of trypomastigote-infected WT and MASP-2-deficient mice. Log-rank test was used to compare survival curves. All data were analyzed with GraphPad Prism software.
Accordingly, both WT and MASP-2-deficient mice survived the acute/high parasitemia phase, with the first cases of mortality presenting only at the later stage of acute trypanosomiasis (i.e. Days 24 and 26 p.i. for WT controls and MASP-2-deficient mice, respectively) (Figure 3B). After 28 days of infection, the percentage of surviving WT and MASP-2-deficient mice was 73% and 83%, respectively. There was no difference whatsoever in the overall long-term survival of both mice groups, with 67% of WT and MASP-2-deficient mice being alive at the end of the experiment (i.e., 82 days p.i.) (Figure 3B).
Our findings described here for the MASP-2-deficient mouse line may not reflect the phenotype of the MBL-null mouse strain previously reported by Rothfuchs and others,33 because complete deficiency in the lectin pathway activity neither resulted in increased parasitemia levels nor higher mortality rates during T. cruzi experimental infection (Figure 3). We therefore conclude that complement activation by the lectin pathway plays a more or less redundant role in the immune clearance of this parasite. Undoubtedly, other mechanisms of mammalian host protection, which does not rely upon downstream activation of complement lectin pathway, may be crucial to prevent host death caused by the high parasitemia burden that characterizes the acute phase of the T. cruzi infection. These may include macrophage-mediated killing of intracellular parasites,38 circulating parasite destruction mediated by the alternative pathway of complement,30 and the generation of lytic antibodies and subsequent activation of complement classical pathway, after a specific antibody response is mounted.39,40
It is worth mentioning that our short-term experiment does not challenge the disease modifying polymorphisms reported in clinical studies on the severity of human trypanosomiasis. The possibility remains that, in long-term disease manifestations, T. cruzi-mediated dilated cardiomyopathy and/or enlargement of the digestive tract, or inherited polymorphic or acquired variations in lectin pathway functional activity, may present as disease modifying determinants in the pathophysiology of human trypanosomiasis. For instance, in a recent large cohort study with patients affected by Chagas' disease, an association between low MASP-2 serum levels and higher risk of chagasic cardiomyopathy was found, suggesting that deficiency in this serine protease could favor disease progression.41 Whether this response can also be observed in the mouse model of complete lectin pathway deficiency remains to be established.
It is also noteworthy that, apart from the T. cruzi Tulahuén strain used in this study, which frequently leads to a lethal acute disease in C57BL/6 mice,36 mammalian infection by genetically distinct parasite strains has been well described,42 which may induce differentially extended complement activation. Therefore, further characterization of the protective activity of the lectin pathway under infection with other virulent T. cruzi strains may be important to understand the relevance of this arm of the immune response against this parasite.
ACKNOWLEDGMENTS
We thank Juana Orellana, Ruth Mora, Nancy Fabres, Paola Wrinkler, and Manuel Sáez for their invaluable expert technical assistance. We are also grateful to Dr. Diego Catalán for critically revising the manuscript.
Footnotes
Financial support: This work was supported by grants 1130099 from Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT) and PIA ACT 112 to AF, and grant G0801952 from the UK Medical Research Council awarded to WJS.
Disclosure: None of the authors has any conflicts of interest.
Authors' addresses: Carolina H. Ribeiro, Carolina Valck, Francisca Noya-Leal, and Arturo Ferreira, Programa Disciplinario de Inmunología, Instituto de Ciencias Biomédicas (ICBM), Facultad de Medicina, Universidad de Chile, Santiago, Chile, E-mails: chager@med.uchile.cl, cvalck@med.uchile.cl, panchinoya@ug.uchile.cl, and aferreir@med.uchile.cl. Nicholas J. Lynch, Cordula M. Stover, and Wilhelm J. Schwaeble, Department of Infection, Immunity, and Inflammation, University of Leicester, Leicester, UK, E-mails: njl12@le.ac.uk, cms13@le.ac.uk, and ws5@le.ac.uk. Youssif M. Ali, Department of Microbiology, Faculty of Pharmacy, Mansoura University, Mansoura, Egypt, E-mail: M_youssif@mans.edu.eg.
References
- 1.Chagas C. Nova tripanozomiase humana: estudos sobre a morfolojia e o ciclo evolutivo do Schizotrypanum cruzi n. gen., n. sp., ajente etiolojico de nova entidade morbida do homem. Mem Inst Oswaldo Cruz. 1909;1:159–218. [Google Scholar]
- 2.Moncayo A. Chagas disease: current epidemiological trends after the interruption of vectorial and transfusional transmission in the Southern Cone countries. Mem Inst Oswaldo Cruz. 2003;98:577–591. doi: 10.1590/s0074-02762003000500001. [DOI] [PubMed] [Google Scholar]
- 3.Tanowitz HB, Weiss LM, Montgomery SP. Chagas disease has now gone global. PLoS Negl Trop Dis. 2011;5:e1136. doi: 10.1371/journal.pntd.0001136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sánchez LV, Ramirez JD. Congenital and oral transmission of American trypanosomiasis: an overview of physiopathogenic aspects. Parasitology. 2013;140:147–159. doi: 10.1017/S0031182012001394. [DOI] [PubMed] [Google Scholar]
- 5.Prata A. Clinical and epidemiological aspects of Chagas disease. Lancet Infect Dis. 2001;1:92–100. doi: 10.1016/S1473-3099(01)00065-2. [DOI] [PubMed] [Google Scholar]
- 6.Machado FS, Dutra WO, Esper L, Gollob KJ, Teixeira MM, Factor SM, Weiss LM, Nagajyothi F, Tanowitz HB, Garg NJ. Current understanding of immunity to Trypanosoma cruzi infection and pathogenesis of Chagas disease. Semin Immunopathol. 2012;34:753–770. doi: 10.1007/s00281-012-0351-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramírez G, Valck C, Aguilar L, Kemmerling U, López-Muñoz R, Cabrera G, Morello A, Ferreira J, Maya JD, Galanti N, Ferreira A. Roles of Trypanosoma cruzi calreticulin in parasite-host interactions and in tumor growth. Mol Immunol. 2012;52:133–140. doi: 10.1016/j.molimm.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 8.Valck C, Ramírez G, López N, Ribeiro CH, Maldonado I, Sánchez G, Ferreira VP, Schwaeble W, Ferreira A. Molecular mechanisms involved in the inactivation of the first component of human complement by Trypanosoma cruzi calreticulin. Mol Immunol. 2010;47:1516–1521. doi: 10.1016/j.molimm.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 9.Ferreira V, Valck C, Sanchez G, Gingras A, Tzima S, Molina MC, Sim R, Schwaeble W, Ferreira A. The classical activation pathway of the human complement system is specifically inhibited by calreticulin from Trypanosoma cruzi. J Immunol. 2004;172:3042–3050. doi: 10.4049/jimmunol.172.5.3042. [DOI] [PubMed] [Google Scholar]
- 10.Ferreira V, Molina MC, Schwaeble W, Lemus D, Ferreira A. Does Trypanosoma cruzi calreticulin modulate the complement system and angiogenesis? Trends Parasitol. 2005;21:169–174. doi: 10.1016/j.pt.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Reid KB, Porter RR. The proteolytic activation systems of complement. Annu Rev Biochem. 1981;50:433–464. doi: 10.1146/annurev.bi.50.070181.002245. [DOI] [PubMed] [Google Scholar]
- 12.Fujita T, Matsushita M, Endo Y. The lectin-complement pathway - its role in innate immunity and evolution. Immunol Rev. 2004;198:185–202. doi: 10.1111/j.0105-2896.2004.0123.x. [DOI] [PubMed] [Google Scholar]
- 13.Matsushita M, Fujita T. Activation of the classical complement pathway by mannose-binding protein in association with a novel C1s-like serine protease. J Exp Med. 1992;176:1497–1502. doi: 10.1084/jem.176.6.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thiel S, Vorup-Jensen T, Stover CM, Schwaeble W, Laursen SB, Poulsen K, Willis AC, Eggleton P, Hansen S, Holmskov U, Reid KB, Jensenius JC. A second serine protease associated with mannan-binding lectin that activates complement. Nature. 1997;386:506–510. doi: 10.1038/386506a0. [DOI] [PubMed] [Google Scholar]
- 15.Stover CM, Thiel S, Lynch NJ, Schwaeble WJ. The rat and mouse homologues of MASP-2 and MAp19, components of the lectin activation pathway of complement. J Immunol. 1999;163:6848–6859. [PubMed] [Google Scholar]
- 16.Dahl MR, Thiel S, Matsushita M, Fujita T, Willis AC, Christensen T, Vorup-Jensen T, Jensenius JC. MASP-3 and its association with distinct complexes of the mannan-binding lectin complement activation pathway. Immunity. 2001;15:127–135. doi: 10.1016/s1074-7613(01)00161-3. [DOI] [PubMed] [Google Scholar]
- 17.Henriksen ML, Brandt J, Andrieu JP, Nielsen C, Jensen PH, Holmskov U, Jorgensen TJ, Palarasah Y, Thielens NM, Hansen S. Heteromeric complexes of native collectin kidney 1 and collectin liver 1 are found in the circulation with MASPs and activate the complement system. J Immunol. 2013;191:6117–6127. doi: 10.4049/jimmunol.1302121. [DOI] [PubMed] [Google Scholar]
- 18.Vorup-Jensen T, Petersen SV, Hansen AG, Poulsen K, Schwaeble W, Sim RB, Reid KB, Davis SJ, Thiel S, Jensenius JC. Distinct pathways of mannan-binding lectin (MBL)- and C1-complex autoactivation revealed by reconstitution of MBL with recombinant MBL-associated serine protease-2. J Immunol. 2000;165:2093–2100. doi: 10.4049/jimmunol.165.4.2093. [DOI] [PubMed] [Google Scholar]
- 19.Thiel S, Petersen SV, Vorup-Jensen T, Matsushita M, Fujita T, Stover CM, Schwaeble WJ, Jensenius JC. Interaction of C1q and mannan-binding lectin (MBL) with C1r, C1s, MBL-associated serine proteases 1 and 2, and the MBL-associated protein MAp19. J Immunol. 2000;165:878–887. doi: 10.4049/jimmunol.165.2.878. [DOI] [PubMed] [Google Scholar]
- 20.Rossi V, Cseh S, Bally I, Thielens NM, Jensenius JC, Arlaud GJ. Substrate specificities of recombinant mannan-binding lectin associated serine proteases-1 and -2. J Biol Chem. 2001;276:40880–40887. doi: 10.1074/jbc.M105934200. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi M, Iwaki D, Kanno K, Ishida Y, Xiong J, Matsushita M, Endo Y, Miura S, Ishii N, Sugamura K, Fujita T. Mannose-binding lectin (MBL)-associated serine protease (MASP)-1 contributes to activation of the lectin complement pathway. J Immunol. 2008;180:6132–6138. doi: 10.4049/jimmunol.180.9.6132. [DOI] [PubMed] [Google Scholar]
- 22.Schwaeble WJ, Lynch NJ, Clark JE, Marber M, Samani NJ, Ali YM, Dudler T, Parent B, Lhotta K, Wallis R, Farrar CA, Sacks S, Lee H, Zhang M, Iwaki D, Takahashi M, Fujita T, Tedford CE, Stover CM. Targeting of mannan-binding lectin-associated serine protease-2 confers protection from myocardial and gastrointestinal ischemia/reperfusion injury. Proc Natl Acad Sci USA. 2011;108:7523–7528. doi: 10.1073/pnas.1101748108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turner MW. The role of mannose-binding lectin in health and disease. Mol Immunol. 2003;40:423–429. doi: 10.1016/s0161-5890(03)00155-x. [DOI] [PubMed] [Google Scholar]
- 24.Thiel S, Frederiksen PD, Jensenius JC. Clinical manifestations of mannan-binding lectin deficiency. Mol Immunol. 2006;43:86–96. doi: 10.1016/j.molimm.2005.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ram S, Lewis LA, Rice PA. Infections of people with complement deficiencies and patients who have undergone splenectomy. Clin Microbiol Rev. 2010;23:740–780. doi: 10.1128/CMR.00048-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Evans-Osses I, de Messias-Reason I, Ramirez MI. The emerging role of complement lectin pathway in trypanosomatids: molecular bases in activation, genetic deficiencies, susceptibility to infection, and complement system-based therapeutics. ScientificWorldJournal. 2013;2013:1–12. doi: 10.1155/2013/675898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cestari I, Evans-Osses I, Schlapbach LJ, de Messias-Reason I, Ramirez MI. Mechanisms of complement lectin pathway activation and resistance by trypanosomatid parasites. Mol Immunol. 2013;53:328–334. doi: 10.1016/j.molimm.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 28.Stengaard-Pedersen K, Thiel S, Gadjeva M, Møller-Kristensen M, Sørensen R, Jensen LT, Sjøholm AG, Fugger L, Jensenius JC. Inherited deficiency of mannan-binding lectin-associated serine protease 2. N Engl J Med. 2003;349:554–560. doi: 10.1056/NEJMoa022836. [DOI] [PubMed] [Google Scholar]
- 29.Sørensen R, Thiel S, Jensenius JC. Mannan-binding lectin-associated serine proteases, characteristics and disease associations. Springer Semin Immun. 2005;27:299–319. doi: 10.1007/s00281-005-0006-z. [DOI] [PubMed] [Google Scholar]
- 30.Cestari I, Krarup A, Sim RB, Inal JM, Ramirez MI. Role of early lectin pathway activation in the complement-mediated killing of Trypanosoma cruzi. Mol Immunol. 2009;47:426–437. doi: 10.1016/j.molimm.2009.08.030. [DOI] [PubMed] [Google Scholar]
- 31.Cestari I, Ramirez MI. Inefficient complement system clearance of Trypanosoma cruzi metacyclic trypomastigotes enables resistant strains to invade eukaryotic cells. PLoS ONE. 2010;5:e9721. doi: 10.1371/journal.pone.0009721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi L, Takahashi K, Dundee J, Shahroor-Karni S, Thiel S, Jensenius JC, Gad F, Hamblin MR, Sastry KN, Ezekowitz RAB. Mannose-binding lectin-deficient mice are susceptible to infection with Staphylococcus aureus. J Exp Med. 2004;199:1379–1390. doi: 10.1084/jem.20032207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rothfuchs AG, Roffê E, Gibson A, Cheever AW, Ezekowitz RA, Takahashi K, Steindel M, Sher A, Báfica A. Mannose-binding lectin regulates host resistance and pathology during experimental infection with Trypanosoma cruzi. PLoS ONE. 2012;7:e47835. doi: 10.1371/journal.pone.0047835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ali YM, Lynch NJ, Haleem KS, Fujita T, Endo Y, Hansen S, Holmskov U, Takahashi K, Stahl GL, Dudler T, Girija UV, Wallis R, Kadioglu A, Stover CM, Andrew PW, Schwaeble WJ. The lectin pathway of complement activation is a critical component of the innate immune response to pneumococcal infection. PLoS Pathog. 2012;8:e1002793. doi: 10.1371/journal.ppat.1002793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Endo Y, Takahashi M, Iwaki D, Ishida Y, Nakazawa N, Kodama T, Matsuzaka T, Kanno K, Liu Y, Tsuchiya K, Kawamura I, Ikawa M, Waguri S, Wada I, Matsushita M, Schwaeble WJ, Fujita T. Mice deficient in ficolin, a lectin complement pathway recognition molecule, are susceptible to Streptococcus pneumoniae infection. J Immunol. 2012;189:5860–5866. doi: 10.4049/jimmunol.1200836. [DOI] [PubMed] [Google Scholar]
- 36.Roggero E, Perez A, Tamae-Kakazu M, Piazzon I, Nepomnaschy I, Wietzerbin J, Serra E, Revelli S, Bottasso O. Differential susceptibility to acute Trypanosoma cruzi infection in BALB/c and C57BL/6 mice is not associated with a distinct parasite load but cytokine abnormalities. Clin Exp Immunol. 2002;128:421–428. doi: 10.1046/j.1365-2249.2002.01874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brener Z. Therapeutic activity and criterion of cure on mice experimentally infected with Trypanosoma cruzi. Rev Inst Med Trop Sao Paulo. 1962;4:389–396. [PubMed] [Google Scholar]
- 38.Vespa GN, Cunha FQ, Silva JS. Nitric oxide is involved in control of Trypanosoma cruzi-induced parasitemia and directly kills the parasite in vitro. Infect Immun. 1994;62:5177–5182. doi: 10.1128/iai.62.11.5177-5182.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krettli AU, Brener Z. Resistance against Trypanosoma cruzi associated to anti-living trypomastigote antibodies. J Immunol. 1982;128:2009–2012. [PubMed] [Google Scholar]
- 40.Gazzinelli RT, Pereira ME, Romanha A, Gazzinelli G, Brener Z. Direct lysis of Trypanosoma cruzi: a novel effector mechanism of protection mediated by human anti-gal antibodies. Parasite Immunol. 1991;13:345–356. doi: 10.1111/j.1365-3024.1991.tb00288.x. [DOI] [PubMed] [Google Scholar]
- 41.Boldt AB, Luz PR, Messias-Reason IJ. MASP2 haplotypes are associated with high risk of cardiomyopathy in chronic Chagas disease. Clin Immunol. 2011;140:63–70. doi: 10.1016/j.clim.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 42.Pena DA, Eger I, Nogueira L, Heck N, Menin A, Báfica A, Steindel M. Selection of TcII Trypanosoma cruzi population following macrophage infection. J Infect Dis. 2011;204:478–486. doi: 10.1093/infdis/jir292. [DOI] [PubMed] [Google Scholar]