Abstract
Perinatal exposure to endocrine disrupting chemicals (EDCs) can induce promiscuous neurobehavioral disturbances. Bisphenol A and phthalates are two widely prevalent and persistent EDCs reported to lead to such effects. Parental and social behaviors are especially vulnerable to endocrine disruption, as these traits are programmed by the organizational-activational effects of testosterone and estrogen. Exposure to BPA and other EDCs disrupts normal maternal care provided by rodents and non-human primates, such as nursing, time she spends hunched over and in the nest, and grooming her pups. Paternal care may also be affected by BPA. No long-term study has linked perinatal exposure to BPA or other EDC and later parental behavioral deficits in humans. The fact that the same brain regions and neural hormone substrates govern parental behaviors in animal models and humans suggests that this suite of behaviors may also be vulnerable in the latter. Social behaviors, such as communication, mate choice, pair bonding, social inquisitiveness and recognition, play behavior, social grooming, copulation, and aggression, are compromised in animal models exposed to BPA, phthalates, and other EDCs. Early contact to these chemicals is also correlated with maladaptive social behaviors in children. These behavioral disturbances may originate by altering the fetal or adult gonadal production of testosterone or estrogen, expression of ESR1, ESR2, and AR in the brain regions governing these behaviors, neuropeptide/protein hormone (oxytocin, vasopressin, and prolactin) and their cognate neural receptors, and/or through epimutations. Robust evidence exists for all of these EDC-induced changes. Concern also exists for transgenerational persistence of such neurobehavioral disruptions. In sum, evidence for social and parental deficits induced by BPA, phthalates, and related chemicals is strongly mounting, and such effects may ultimately compromise the overall social fitness of populations to come.
Keywords: EDC, bisphenol A, phthalate, xenoestrogen, rodent models, brain development, epigenetics, neuropeptides
Introduction
The perinatal environment can dramatically shape later adult behaviors, and these disruptions can be propagated through transgenerational transmission to future generations (Rosenfeld, 2014). The impact of varying parental investment on offspring brain development is also gaining interest (Dulac et al., 2014; Rilling and Young, 2014). Disturbances in parental behaviors, such as nursing/feeding, huddling over, and grooming the neonates can have dramatic epigenetic and phenotypic consequences that persist for generations to come (Weaver et al., 2006).
Due to underlying neural disruptions, parental and social behaviors can be impacted by developmental exposure to endocrine disrupting chemicals (EDCs). Strong conservation exists in brain development and function across species spanning from rodents to humans (Rice and Barone, 2000; Howdeshell, 2002). In animal models and humans, neurobehavioral development is vulnerable to EDCs, because of the organizational-activational of endogenous steroidogenic hormones.
One of the most preeminent discoveries in neuroendocrinology in the last century was that sex steroid hormones, testosterone and estrogen, guide perinatal brain development in a sex dependent manner (Phoenix et al., 1959; Arnold and Breedlove, 1985; Morris et al., 2004). This early brain programming is termed the “organizational effects” of these steroid hormones. However, full elaboration of many sex-dependent behaviors requires a later surge of testosterone in adult males (“activational effects”). The organizational-activational effects of testosterone in many brain regions are due to aromatization to estrogen (Watson and Adkins-Regan, 1989; Konkle and McCarthy, 2011). Exposure to EDCs that disrupt these normal steroidogenic effects may thus result in neurobehavioral deficits, including social and parental behavioral deficits.
The two most common EDCs associated with parental and social behavioral disturbances are bisphenol A (BPA) and phthalates. Therefore, these two will be the primary focus, although, where appropriate, effects of other EDCs on these traits will be discussed. BPA is one of the most widely prevalent EDCs with production currently reported to be in excess of 15 billion pounds per year (Vandenberg et al., 2013; Grandviewresearch, 2014). BPA is present in a wide variety of commonly used products and applications, including polycarbonate plastics, the lining of metal food cans, certain dental sealants, thermal receipt paper, and many other items that are currently not required to be labeled that they contain BPA. The abundance of BPA has ensured extensive and longstanding exposure of animals and humans, including pregnant women (Environment Canada, 2008; Vandenberg et al., 2013). There is strong evidence that BPA is a neuroendocrine disruptor (Leon-Olea et al., 2014).
Di(2-ethylhexyl) phthalate (DEHP) is another ubiquitous EDC present in plastic products personal care products, paints, pharmaceuticals, food products, and textiles (Latini et al., 2003). Similar to BPA, this chemical can leach out of plastic products when heated. Its primary metabolite, mono-ethylhexyl-phthalate (MEHP) can also result in neuroendocrine disruption (Leon-Olea et al., 2014).
This review will first consider the evidence that BPA, phthalates, and other EDCs can alter parental care provided by the mother and the scant evidence to date by the father. Next, we will consider the impact of developmental exposure to these chemicals on social behaviors in animal models and linkages in humans. Finally, the potential mechanisms by which EDCs may affect these behavioral patterns will be explored.
Evidence of endocrine disruption of parental behaviors
There have been no long-term studies examining for potential linkage of early exposure to these chemicals and later parental behavior deficits in humans. Therefore, this section will focus on the existing evidence from rodent and other animal models (Summarized in Table 1). A handful of rodent studies have reported that early exposure to EDCs can result in later effects on maternal care. General maternal behaviors assessed in these collective studies include the amount of time the female spends nursing, hunched over and in the nest, grooming, and latency to retrieve her pups.
Table 1.
Animal model and human epidemiological studies linking bisphenol A (BPA) and phthalates exposure to parental and social behavioral changes.
| Publication(s) | Animal model/human cohort population | EDC(s) tested or correlation analysis performed | Dosing regimen/method to measure BPA concentrations | Major findings |
|---|---|---|---|---|
| PARENTAL BEHAVIORS | ||||
| Palanza et al., 2002 | CD1 female mice | BPA | Prenatal exposure of F1 offspring to 10 μg BPA/kg body weight (bw)/day or oil by oral administration through the F0 dam from days 14 to 18 of gestation. Some F1 adults (2–2.5 months of age) were also treated with 10 μg BPA/kg bw/day or oil and treatments spanned from days 14 to 18 of gestation. There were thus those F1 offspring who only received prenatal or adult exposure to BPA and those that were exposed to this chemical during the prenatal and adult periods resulting in four different treatment groups. | • F1 females exposed to BPA during the prenatal or adult period spent spend less time nursing and huddling over the pups but greater time engaged in nest building than controls and those that received BPA during both time periods. |
| Della Seta et al., 2005 | Sprague-Dawley female rats | BPA | Adult F0 exposure to 40 μg BPA/kg bw/day by oral administration from the day after mating (gestation) through lactation (42 day duration). |
|
| Boudalia et al., 2014 | Wistar female rats | BPA | F0 adult exposure to 5 μg BPA/kg bw/day orally from the first day of gestation (GD1) through lactation (Post-natal day, PND 21). |
|
| F1 offspring then treated every two days with same dosing solution as received by the F0 mother from weaning (PND 21) until mating at adulthood (PND 100). | ||||
| Kundakovic et al., 2013 | BALB/c mice | BPA | Adult exposure of F0 dams to one of the three oral doses of BPA (2, 20, 200 μg/kg bw/day) from gestational days 0 to 19. |
|
| Nakagami et al., 2009 | Cynomolgus monkeys (Macaca fascicularis) | BPA | Male F1 infants exposed from gestational day 20 through term to 10 μg BPA/kg bw/day via osmotic pump. |
|
| SOCIAL BEHAVIORS: ANIMAL MODELS | ||||
| Williams et al., 2013 | California mice (Peromyscus californicus) | BPA | Two weeks prior to breeding of the F0 dam through weaning at PND 30, they were exposed to 50 mg BPA/kg feed weight in the diet. |
|
| F1 males and females were placed on a control diet at weaning through adulthood. | ||||
| Ward and Blum, 2012 | Native blacktail shiner fish (Cyprinella venusta) and introduced red shiner fish (C. venusta) | BPA | Adult F0 exposure for 14 days to BPA 1280 μg/L water (5.6 μM). |
|
| Jasarevic et al., 2011 | Deer mice (P. maniculatus bairdii) | BPA | Two weeks prior to breeding of the F0 dam through weaning at PND 21, they were exposed to 50 mg BPA/kg feed weight in the diet. |
|
| F1 males and females were placed on a control diet at weaning through adulthood. | ||||
| Razzoli et al., 2005 | Pair-bonded monogamous Mongolian gerbils (Meriones unguiculatus) | BPA and EE2 | Adult F0 females treated daily with oral administration of BPA (2 or 20 μg/kg bw/day) or EE2 (0.04 μg/kg bw/day) from day of pairing to day 21 of cohabituation. | • BPA and EE2 adult exposed F0 females showed increase investigation of their F0 control male partner and reduced exploration. |
| Wolstenholme et al., 2013 | C57Bl/6 mice | BPA | Prenatal exposure (7–10 days prior to F0 female being paired with a breeder male and for a 2 week duration) and ancestral exposure to 5000 μg/kg fw through the F0 maternal diet. |
|
| Porrini et al., 2005 | Sprague-Dawley rats | BPA | Daily oral administration of 4000 μg BPA/kg bw/day to adult F0 dams from gestation through lactation (Mating through weaning of pups at PND 21). F1 females were not exposed to BPA after weaning. |
|
| Dessi-Fulgheri et al., 2002 | Sprague-Dawley rats | BPA | Perinatal exposure of F1 females to BPA either at 40 mg/kg bw/day via daily maternal (F0) oral dosing from conception to weaning or 400 mg/kg bw/day from gestational day 14 to postnatal day 6. |
|
| Wolstenholme et al., 2011b | C57Bl6J mice | BPA | Gestational exposure of F1 offspring to 1250 μg BPA/kg fw in the maternal diet. | • Gestational exposure of both F1 sexes resulted in increased play solicitations and approaches. |
| Jones et al., 2011 | Long-Evans rats | BPA | Gestational (gestational day 7 to PND 14) and lactational exposure of F1 offspring to 50 μg/kg bw/day through daily maternal oral administration. |
|
| Monje et al., 2009 | Inbred Wistar-derived rats | BPA | Neonatal exposure (PND 1–7) of F1 pups to 50 μg/kg bw/day or 20,000 μg/kg bw/day via daily subcutaneous injections. |
|
| Panzica et al., 2005 | Male Japanese quail (Coturnix japonica) | BPA | 50, 100, and 200 μg BPA per egg. |
|
| Estradiol benzoate (EB) | 10 or 25 μg EB per egg. | |||
| DES | 700 ng DES per egg. | |||
| Genistein | 1000 μg genistein per egg. | |||
| Wibe et al., 2002; Kaplan et al., 2013 | Threespine stickleback (Gastrosteus aculeatus) and Mummichog (Fundulus heteroclitus) | Benzyl Butyl Phthalate (BBP) | Adult F0 exposure to 100 μg/L in the water (0.32 μM) daily for 26 days (threespine stickleback) or 4 weeks (mummichog). |
|
| Betz et al., 2013 | Sprague-Dawley male rats | BPP | Five to six week old F0 males were provided 5000 and 10,000 mg/L in the drinking water (0.16 and 0.032 μM) until they were 20–21 weeks of age (15 week duration of exposure). |
|
| Lee et al., 2006 | Wistar-Imamichi rats | Di-n-butyl phthalate (DBP) | Perinatal exposure of F1 offspring through the maternal diet from gestational day 15 to weaning (PND 21) to: DBP- 20, 200, 2000, and 10,000 mg/kg bw/day DINP- 40, 400, 4000, and 20,000 mg/kg bw/day DEHA- 480, 2400, and 12,000 mg/kg bw/day |
|
| Diisononyl phthalate (DINP) | ||||
| Di-2-ethylhexyl adipate (DEHA) | ||||
| SOCIAL BEHAVIORS: HUMAN EPIDEMIOLOGICAL STUDIES | ||||
| Perera et al., 2012 | 87 boys and 111 girls spanning 3–5 years of age | Maternal urinary BPA concentrations | Median maternal urinary concentrations at 34 weeks gestation: 1.8 μg/L (7.9 nM) Median child urinary concentrations at 3–4 years of age: 3.5 μg/L (15.3 nM) |
|
| Evans et al., 2014 | 77 boys and 76 girls spanning 6–10 years of age | Maternal urinary BPA concentrations | Median maternal urinary BPA from 10 to 39 weeks gestation with mean gestational age at collection = 26.6 weeks: 1.1 μg/L (4.8 nM) | • Increased maternal urinary BPA concentrations linked with greater externalizing and aggressive behaviors in boys. |
| Braun et al., 2009 | 249 mothers and their 2-year old children | Maternal urinary BPA concentrations | Median maternal urinary concentrations: 1.3–1.8 μg/L (5.6–7.9 nM), as measured at 16 and 26 weeks gestation and at birth | • Linkage of prenatal BPA concentrations (as determined by maternal urinary concentrations during pregnancy) and externalizing behaviors for girls but not boys. |
| Miodovnik et al., 2011 | 404 mothers and their 7–9 years of age children | Maternal urinary BPA and 10 individual phthalate metabolite concentrations | Maternal urine was collected between 25 and 40 weeks gestation (mean = 31.2 weeks). |
|
| Median BPA concentration: 1.3 μg/L (5.6 nM) | ||||
| Median phthalate concentrations ranged from 1.6 to 430 ng/ml | ||||
| Lien et al., 2014 | 122 mothers and their 8 year old children | Maternal urinary phthalate concentrations (7 different forms) | Maternal urine was collected in the third trimester of gestation. Geometric mean range of urinary phthalate metabolite concentrations: 26.8–109.1 μg/g creatinine | • Positive correlation with perinatal exposure to phthalates and delinquent and aggressive behavioral scores. |
| Kobrosly et al., 2014 | 153 mothers and their 6–10 year old children | Maternal urinary phthalate concentrations (monoisobutyl phthalate, MiBP, and monobenzyl phthalate, MBzP) | Maternal urine was collected between 10 and 39 weeks gestation (mean = 26.6 weeks) |
|
| Median of MiBP = 1.0 ng/ml (4.5 pM) | ||||
| Median of MBzP = 3.4 ng/ml (13.3 pM) | ||||
CD-1 female mice exposed orally during the fetal and/or adult period to BPA (10 μg/kg body weight (bw)/day for gestational days 14–18) spend less time nursing their pups and huddling over them while in the nest (Palanza et al., 2002). Acute exposure of adult female Sprague-Dawley rats exposed to BPA (40 μg/kg bw, orally per day) from mating to weaning of their pups disrupts both active and passive maternal care, manifested by decreased time licking and grooming the pups, a trend to less ano-genital licking, and less time assuming an arched-back posture to allow the pups to suckle (Della Seta et al., 2005). Similarly, Wistar female rats treated with 5 μg BPA/kg bw/day from the first day of gestation through lactation (postnatal day, PND 21) decreased the amount of time they nursed and attended to their pups (Boudalia et al., 2014). Another study tested the effects of gestational exposure (0–19 days) to three oral doses of BPA (2, 20, and 200 μg/kg bw/day) on maternal care provided by BALB/c mice. In contrast to the prior studies, the highest dose of BPA tested increased the amount of time females spent licking and grooming and nursing their pups in an arched back position (Kundakovic et al., 2013).
These conflicting rodent model findings may be attributed to various factors. First, all four of these above studies used rats or mice of different strains, and there is evidence of strain-specificity and phenotypic differences in response to BPA and other estrogenic EDC (Spearow et al., 1999, 2001; Kendziorski et al., 2012). The administered dose and timing, duration of exposure (perinatal vs. adult), and corresponding generational differences (F0 vs. F1) may also be potential factors. Finally, differences in animal husbandry (composition of the cages and water bottles, diet-phytoestrogen or non-phytoestrogen free, and shavings-corn cob or aspen) might account for the disparate rodent results.
Infants may influence, through vocalizations, direct contact, and other forms of communication, the amount of parental care provided to them with males tending to initiate and receive more parental investment (Della Seta et al., 2005; Hao et al., 2011). Early EDC exposure though may alter an infant's ability to stimulate maternal care provided to them. For instance, the behavioral patterns of Cynomolgus monkeys (Macaca fascicularis) male infants prenatally (gestational day 20 through term) exposed to BPA (10 μg/kg bw/day via osmotic pump) were more reminiscent of females, and these mothers nursed their “feminized” sons less than those rearing control males (Nakagami et al., 2009).
While there are no studies to date on whether acute and developmental exposure to phthalates affects maternal care, other EDCs have been reported to disrupt these behavioral patterns. Long-Evans (LE) rat pups prenatally exposed through their biological dam to the environmental chemical 3, 4, 3′, 4′-tetrachlorobiphenyl (PCB 77, 2000 μg/kg bw/day on gestational days 6 through 18 via daily subcutaneous injections) resulted in their foster dams spending an increased frequency nursing them and allogrooming (Cummings et al., 2005). This cross-foster approach further revealed the complex interactions exist between maternal and fetal exposure to PCB 77 and amount of time foster dams spent in the nest and grooming the pups, along with decreased duration engaged in high-crouch nursing. Perinatal exposure (either 3 days after the dam was paired with a male or 3 days after parturition of a previous litter through weaning of pups) via daily maternal oral dosing to diethylstilbestrol (DES, 0.2 μg/kg bw/day) or methoxychlor (MXC, 2000 mg/kg bw/day) of monogamous female pine voles did not however affect their later maternal care behaviors (Engell et al., 2006).
The impact of EDCs on later paternal care provided is largely unknown, probably because few mammalian species exhibit biparental care (Clutton-Brock, 1989). Yet, disturbances in paternal care may have dramatic epigenetic and phenotypic consequences that persist for several generations (Bredy et al., 2007; Braun and Champagne, 2014; McGhee and Bell, 2014). Even so, there have been no published rodent studies to date on how early exposure to EDCs affects paternal care. However, in polygynous sand gobies (Pomatoschistus minutus), where the male is responsible for tending the eggs, endocrine disruption of paternal care behaviors has been reported (Saaristo et al., 2010). Polygynous sand goby males compete for females and then assume responsibility for nest building and attendance. However, adult males exposed for 1–4 weeks to 11 ng/L water (36.7 pM) of 17α-ethinyl estradiol (EE2, estrogen in birth control pills) showed suppressed nest building activity, along with decreased courtship behaviors. These combined deficiencies thus likely effect successful fry rearing by exposed males. Future work is needed in monogamous and biparental rodent and other animal models to determine how developmental exposure to EDC affects paternal care and parenting providing by his partner.
Evidence that endocrine disruptors affect social behaviors
Extensive data from retrospective human studies and animal models links perinatal exposure to EDCs and later social deficits (Table 1). The evidence in animal models will first be considered. Social behaviors to be considered include any that involve an interaction between difference members of the same species, such as various forms of communication, mate choice, pair bonding, social inquisitiveness and recognition, play behavior, social grooming, copulation, and aggression.
Animal models
Animal communication assumes various forms besides direct vocalizations. In California mice (Peromyscus californicus), males use territorial marking to communicate and protect their home range and mate from intruders. Males pericoceptionally through perinatally exposed (2 weeks prior to breeding of the dam through weaning at PND 30) to BPA (50 mg/kg feed weight in the maternal diet) show reduction of this behavior when a control male is present in the testing arena (Williams et al., 2013). BPA exposure (1280 μg/L water, 5.6 μM for 14 days) of native blacktail shiner fish (Cyprinella venusta) and introduced red shiner fish (C. venusta) disrupts normal visual communication signals and abolishes the species-dependent sexually selected behavioral traits with the net potential for hybridization to occur between these two otherwise isolated species (Ward and Blum, 2012). Sprague-Dawley female rats prenatally exposed from gestational days 16 to 18 via daily intraperitoneal injection to the PCB mixture Aroclor (A) 1221 (100 μg/kg, 1000 μg/kg, and 10,000 μg/kg bw/day) have later vocalization deficits, as well as changes in mating patterns, movement and likelihood to mate (Steinberg et al., 2007).
Mate choice and pair-bonding formation are vulnerable to endocrine disruption. In mate choice trials, control and BPA-exposed female deer mice (P. maniculatus bairdii) selectively reject males developmentally exposed to BPA (50 mg/kg feed weight through the maternal diet) (Jasarevic et al., 2011). Likewise, female F3 Sprague-Dawley rats prefer F3 males whose ancestors were not prenatally exposed to the anti-androgenic EDC, vinclozolin (100 mg/kg bw/day from gestational days 8 to 14 via daily intraperitoneal injection) (Crews et al., 2007). Female sand gobies favor mating with control males, as opposed to those exposed for 7–24 days to EE2 (4 ng/L water, 13.3 nM) (Saaristo et al., 2009). Pair-bonded monogamous Mongolian gerbil (Meriones unguiculatus) females treated for a 3 week duration (from pairing to day 21 of cohabituation) with daily oral administration of either BPA (2 or 20 μg/kg bw/day) or EE2 (0.04 μg/kg bw/day) exhibit heightened investigation of their partner and less exploration than untreated controls (Razzoli et al., 2005).
Introduction of new animals into the habitat results in social inquisitiveness and in time recognition of the new member with reduced bouts investigating this individual, i.e. habituation. Developmental and transgenerational exposure to BPA (5000 μg/kg feed weight through the F0 maternal diet 7–10 days prior to being paired with a breeder male and for 2 weeks thereafter) prolonged the duration of time juvenile F1 and F3 C57BL/6 mice, respectively, spend investigating a novel animal (Wolstenholme et al., 2013). Further, F3 females, whose ancestors were exposed to BPA, persisted in investigating novel females longer than controls, suggestive of impaired dishabituation.
Exposure to BPA may affect other socio-sexual behaviors, including play, social investigation, copulation, and aggression. Decrease amount of play with males and social grooming were evident in female Sprague-Dawley rats perinatally exposed to BPA (4000 μg/kg bw/day to the dams via daily oral administration from mating through weaning at PND 21), which indicates that this chemical can defeminize select female social behaviors (Porrini et al., 2005). Sprague-Dawley rats exposed perinatally to BPA (40 mg/kg bw/day from conception to weaning or 400 mg/kg/day from gestational day 14 to postnatal day 6 via daily maternal oral dosing) demonstrated masculinization of female social behaviors with such females selectively preferring to play with and engage in socio-sexual exploration of other females (Dessi-Fulgheri et al., 2002). Another study though with C57Bl6J mice suggests that gestational exposure to 1250 μg BPA/kg feed weight increased play solicitations and approaches by both sexes (Wolstenholme et al., 2011b).
Sexual behaviors are also vulnerable to the early effects of BPA. Male LE rats, who received a “low dose” BPA (50 μg/kg bw/day through maternal oral administration spanning gestation and lactation (gestational day 7 through PND 14), were sexually incompetent as adults, but no deficits in female sexual behavior were noted in this study (Jones et al., 2011). In contrast, another report found that neonatal exposure of inbred Wistar-derived female rats to BPA (50 μg/kg bw/day or 20,000 μg/kg bw/day via daily subcutaneous injections to the pups) had later reductions in proceptive behaviors (Monje et al., 2009). While BPA exposure (50, 100, and 200 μg per egg) did overtly effect male Japanese quail (Coturnix japonica) copulatory behaviors, in ovo treatment with other estrogenic chemicals, including estradiol benzoate (10 or 25 μg per egg), DES (700 ng per egg), and the soy phytoestrogen, genistein (1000 μg per egg), abolished this behavior in pubertal males (Panzica et al., 2005).
Current data demonstrate that phthalates can also affect varying social behaviors in rodents and fish. Benzyl butyl phthalate (BBP) exposure through the water (100 μg/L, 0.32 μM) altered the shoaling behaviors (collection of fish for social purposes) in threespine stickleback (Gastrosteus aculeatus), who were treated each day for 26 days, and mummichog, a small killifish, (Fundulus heteroclitus), who were treated daily for 4 weeks (Wibe et al., 2002; Kaplan et al., 2013). BPP-treated threespine stickleback fish tended to aggregate in a single shoal that remained at the bottom of the test aquarium; whereas mummichog exposed fish preferred to shoal with smaller size fish. Male Sprague-Dawley rats exposed from adolescence to adulthood (5–6 weeks of age to 20–21 weeks of age) to BPP through the drinking water (5000 μg/L and 10, 000 μg/L or 0.016 and 0.032 μM, respectively) displayed aberrant social behaviors (Betz et al., 2013). Male and female Wistar-Imamichi rats developmentally exposed through the maternal diet from gestational day 15 through weaning (PND 21) to various phthalate chemicals (di-n-butyl phthalate, DBP- 20, 200, 2000, and 10,000 mg/kg bw/day; diisononyl phthalate, DINP- 40, 400, 4000, and 20,000 mg/kg bw/day; di-2-ethylhexyl adipate, DEHA- 480, 2400, and 12,000 mg/kg bw/day) exhibited sexual behavioral deficits (Lee et al., 2006). Copulatory behavior was diminished in male rats exposed to the varying doses of these chemicals. Similarly, the lordosis quotient was reduced in all treatment group females.
Human epidemiological studies
Associations between early BPA exposure and social behavioral disruptions in children are generally based on urinary BPA concentrations in the mother or child (Table 1). An African-American and Dominican women and their children population cohort that included 87 boys and 111 girls spanning 3–5 years of age showed higher exposure of BPA correlated with increased aggressive behaviors in boys but decreased aggression in girls (Perera et al., 2012). A more recent study with 153 six to ten year old children (77 boys and 76 girls) also demonstrated similar sex differences in vulnerability with increased maternal urinary BPA concentrations linked with greater externalizing and aggressive behaviors in boys (Evans et al., 2014). However, a study examining 249 mothers and their 2-year old children reported elevated prenatal BPA exposure associated with greater externalizing (aggression and hyperactivity) in girls but not boys (Braun et al., 2009).
Perinatal exposure to phthalates is also correlated with social behavioral disturbances. A study population of 404 mothers and their 7–9 years of age children pairs linked early contact to this chemical and later childhood social impairments, including social cognition, communication, and awareness (Miodovnik et al., 2011). This study however did not find any association between BPA exposure and effects on the examined social behaviors or sex-dependent differences. Examination of 122 mother-8 year old child pairs from Taiwan showed positive correlation of delinquent and aggressive behavioral scores and early exposure to different phthalate chemicals, as evidenced by maternal urinary concentrations (Lien et al., 2014). However, this study did not examine for potential sex-dependent differences. Another study with 153 mothers and their 6–10 year old children indicates that prenatal phthalate exposure is associated with later sex-dependent behavioral disturbances (Kobrosly et al., 2014). Boys of mothers with higher urinary monoisobutyl phthalate concentrations were more likely to show higher scores for inattention, rule-breaking behavior, aggression, and conduct problems; whereas increased concentration of monobenzyl phthalate was correlated with conduct problems in boys but reduced anxiety scores in girls.
Mechanisms by which endocrine disruptors may affect parental and social behaviors
Disruption of steroid hormone production or at the steroid receptor level
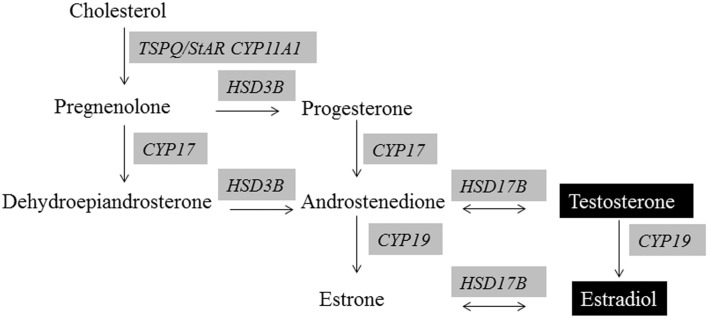
As detailed above, full elaboration of many social and parental behaviors is dependent on the organizational and activational effects of testosterone and estrogen (Phoenix et al., 1959; Arnold and Breedlove, 1985; Morris et al., 2004). Figure 1 illustrates the primary factors and enzymes required for normal testosterone and estrogen production. Both BPA and phthalates can affect steroidogenesis at several points in the pathway for males and females. Steroidogenic acute regulatory protein (StAR, STARD1) is an essential mitochondrial protein for transporting cholesterol into the cell and is thus considered the rate-limiting step for steroid hormone production. BPA suppresses StAR mRNA expression in male and female gonads of rodents and fish (D'Cruz et al., 2012; Horstman et al., 2012; Liu et al., 2012; Peretz and Flaws, 2013; Savchuk et al., 2013). Expression of gonadal steroidogenic enzymes, including Cyp11, Hsd3b, Hsd17b, Cyp17, Cyp19 is also generally inhibited by BPA exposure (D'Cruz et al., 2012; Liu et al., 2012; Nanjappa et al., 2012; Peretz and Flaws, 2013; Savchuk et al., 2013).
Figure 1.
Steroidogenesis of androgens and estrogens. Evidence exists that all of the shaded enzymes required in the synthesis of these hormones are altered by BPA and/or phthalate exposure.
Most animal model studies report BPA exposure decreases production of testosterone (T) and estrogen (E2) by male and female gonads (Akingbemi et al., 2004b; Peretz et al., 2011; D'Cruz et al., 2012; Nanjappa et al., 2012). However, a study with isolated porcine ovarian follicles suggests potential dose-dependent effects with lower dose BPA concentrations (0.1 mM) elevating E2; whereas large doses (1 and 10 mm) suppressing E2 levels (Grasselli et al., 2010). In ovo BPA treatment (0.01 or 1.4 ppm) of Caiman latirostris eggs elevated E2 and lowered T concentrations and concomitantly reversed the normal temperature sex determination mechanisms with a predominance of females occurring in these groups (Stoker et al., 2008). In girls with precocious puberty, elevated urinary BPA concentrations were associated with elevated T, E2, and pregnenolone levels (Lee et al., 2014). Phthalate exposure is also associated with decreased StAR and steroidogenic enzyme expression and steroid hormone production (T and E2) by the male gonad (Akingbemi et al., 2001, 2004a; Svechnikov et al., 2008; Botelho et al., 2009; Chauvigne et al., 2011; Desdoits-Lethimonier et al., 2012; Saillenfait et al., 2013; Beverly et al., 2014), as well as circulating concentrations of T and E2 in men (Meeker et al., 2009). In contrast, one report identifying disrupted social interactions in phthalate-treated rats indicated that this chemical increased E2 concentrations (Betz et al., 2013).
BPA and phthalates may also disrupt normal organizational/activational steroidogenic effects by altering the expression of the cognate receptors, ESRs and AR, in the neural regions governing social and parental behaviors, namely the hypothalamus. In this region, developmental exposure to BPA has been shown to alter the expression of ESR1 and ESR2 in rodent and ovine models, although the directionality is seemingly dependent upon sub-region, dose, time of assessment, and possibly species (Ramos et al., 2003; Ceccarelli et al., 2007; Monje et al., 2007; Mahoney and Padmanabhan, 2010; Cao et al., 2012, 2013, 2014; Patisaul et al., 2012; Kundakovic et al., 2013). Scant information exists on how phthalates may affect neural expression of ESRs and AR. The aforementioned rat phthalate study above also showed increased ESR1 expression in the amygdala of rats treated with 5 mg/L (0.16 μM) BBP (Betz et al., 2013). Combined treatment of 4 wk old rats with BPA (285.4 mg/kg) and DBP (285.4 mg/kg) in the feed increased AR expression in the brain (Zhang et al., 2013), which might render these animals more sensitive to endogenous and exogenous androgens.
Disruption of the hypothalamic-pituitary-adrenal (HPA) axis
BPA, phthalates, and other EDC exposure may disturb parental and social behaviors through disruptions in the classic stress-associated adrenocortical axis. One rat study showed that early BPA exposure (oral administration of 40 μg/kg bw/day to the dam throughout gestation and lactation) led to sex dependent effects on circulating corticosterone concentrations (elevated in BPA-exposed females) and neural (hippocampal expression) of Gr (lower expression in BPA-exposed males) (Poimenova et al., 2010). Further, BPA-treated female rats developmentally exposed to this same dosing regimen exhibited increased basal corticosterone and suppressed hypothalamic Gr expression (Panagiotidou et al., 2014).
Another study in rats where dams were subcutaneously injected with 2 μg BPA/kg bw/day from gestational day 10 to lactational day 7 showed sex-dependent alterations in the adreno-cortical axis (Chen et al., 2014). BPA-exposed male rats had increased basal concentrations of serum corticosterone and adrenocorticotropin (ACTH) and corticotropin-releasing hormone (Crh) mRNA expression, but these changes were not evident in exposed females. Following subsequent exposure to a mild stressor, corticosterone and ACTH concentrations increased further in BPA males; whereas, these hormones decreased in BPA females. In non-stressed animals, Gr mRNA was increased in the hippocampus and hypothalamic paraventricular nucleus of BPA females. In contrast, these transcripts decreased in BPA males. The collective results suggest that BPA exposure might affect the HPA axis in males and females but the specific effects are sex-dependent.
Phthalates also appear to disrupt the HPA axis. Oral exposure to DEHP (500 mg/kg bw/day for 4 days) increased concentrations of ACTH and corticosterone in treated male rats at 20 and 40 days of age; whereas no effects were observed in adult (60 days of age) males (Supornsilchai et al., 2007). Isolated adrenocortical cells from the 20 and 40 day old treated rats were more sensitized to the effects of ACTH, dibutyrl cAMP, and 22R-hydroxylase, as evidenced by increased production of corticosterone and ACTH treatment stimulated greater transport of endogenous cholesterol into the mitochondria.
Disruption of neuropeptide/protein hormones and their cognate receptors
In the mouse and rat, central oxytocin receptors (OXTR) are obligatory for the expression of maternal behavior, and variation in OXTR levels in the medial preoptic area of the hypothalamus (MPOA) are functionally linked to differences in degree of maternal care (Champagne et al., 2006; Weaver, 2007). California mice fathers are known to exhibit increased oxytocin immunoreactivity compared to non-paternal males (Lambert et al., 2011). Increased signaling through the OXTR is correlated with high degree of maternal behavior, such as pup licking and grooming, while ESR1 is an important regulator of OXTR expression (Champagne et al., 2006). Prolactin, oxytocin, and vasopressin have been implicated in paternal care in California mice, monogamous prairie voles (Microtus ochrogaster), and humans (Gubernick and Nelson, 1989; Parker and Lee, 2001; Bales et al., 2004; Wynne-Edwards and Timonin, 2007; Gordon et al., 2010). Social behaviors are also dependent upon oxytocin, vasopressin, and protein binding to their cognate receptors in the brain (Carlson et al., 2006; Benarroch, 2013; Walker and McGlone, 2013; Wudarczyk et al., 2013; Babb et al., 2014; Lieberwirth and Wang, 2014).
Brains from 18.5 days post-coitus (dpc) mice exposed to BPA express less oxytocin, OXTR, and vasopressin than control males (Wolstenholme et al., 2011a, 2012). Reductions in brain expression for vasopressin persists in transgenerationally BPA exposed F4 males and females, and oxytocin is decreased in F4 males (Wolstenholme et al., 2012). Another study, however, suggests that in the paraventricular nucleus (PVN), oxytocin-immunnoreactive neurons increase in neonatally exposed BPA female Long Evans rats (Adewale et al., 2011), but non-pregnant, non-lactating females were examined. A recent study with juvenile prairie voles showed neonatal exposure to BPA increased AVP-immunoreactive neurons in the anterior PVN but decreased OT-immunoreactive neurons in this same region (Sullivan et al., 2014). In vivo and in vitro studies suggest that BPA increases PRL levels in male and female rats (Steinmetz et al., 1997; Goloubkova et al., 2000; Ramos et al., 2003; Delclos et al., 2014). Likewise, phthalate exposure is associated with elevated prolactin production in rats of both sexes and men (Lee et al., 2004; Li et al., 2011). No previous study has assessed the effects of BPA or phthalate-exposure on PRL in parenting males and females.
Epimutations
Epimutations are the most plausible mechanisms by which early exposure to BPA, phthalates, and other EDCs may lead to later neurobehavioral disturbances. Such changes may include alteration in DNA methylation, histone proteins, non-coding RNA, or chromatin arrangement. It is now apparent from a variety of animal model studies that BPA, phthlates, and other EDCs can lead to DNA methylation and corresponding gene expression changes in a variety of tissues, including the brain (Yaoi et al., 2008; Jang et al., 2012; Tang et al., 2012; Kundakovic et al., 2014; Zhao et al., 2014; Martinez-Arguelles and Papadopoulos, 2015).
Only two studies to date have shown BPA-induced DNA methylation changes in the brain. Developmental exposure to BPA alters the DNA methylation promoter state in many genes of the mouse forebrain (Yaoi et al., 2008). Persistent DNA methylation changes are evident in one of the promoters of Bdnf in the hippocampus and cord blood in female and male mice subjected to prenatal exposure to BPA with hypemethylation apparent in females but hypomethylation observed in males (Kundakovic et al., 2014). DNA methyl transferase (DNMT) and methyl CPG binding proteins (MECP) guide global DNA methylation changes.
BPA may simultaneously effect DNA methylation and histone protein modifications. For instance, exposure to BPA suppresses rat brain cortical expression of the ion transporter (Kcc2) through both DNA methylation via MECP2 and histone protein (H3K9) binding to this gene (Yeo et al., 2013). Phthalates can also induce histone protein modifications in neuronal cells, as evidenced by phthalate-induced Sp3 suppression associated with deacetylation (via HDAC4) and ensuing polyubiquination in neuroblastoma cells (Guida et al., 2014).
The expression of Dnmt1, Dntm3a, Dnmt3b, and Mecp2 is altered in various brain regions, including basolateral amygdala, cortex, and hypothalamus, in BPA-treated rodent models (Kundakovic et al., 2013; Warita et al., 2013; Zhou et al., 2013). The histone protein modification enzyme (HDAC2) is up-regulated in the hippocampus of adult males exposed to BPA (Zhang et al., 2014a). The histone methyltransferase enzyme (EZH2) and histone H3 trimethylation is upregulated in the mammary gland of mice exposed to BPA in utero and MCF-7 cells treated with this chemical (Doherty et al., 2010).
While no study has determined whether BPA exposure affects non-coding (nc) RNAs (including microRNAs, miRNAs) in the brain, there is evidence that this chemical can affect expression of these biomolecules in other cells, including MCF7 (Tilghman et al., 2012), ovarian (Veiga-Lopez et al., 2013), and placental cells (Avissar-Whiting et al., 2010). No study to date has assessed whether phthalate exposure affects ncRNAs in the brain or other regions.
Conclusions
Strong evidence exists that parental and social behaviors in a wide variety of species, including by translation humans, are vulnerable to perinatal exposure to EDCs, including BPA and phthalates. These effects are likely multifactorial in origin, but the net result is presumably that these chemicals disrupt normal organizational and activational programming of the brain. EDC-induced disruptions on neural programming may occur as a result of alterations in fetal or adult steroid hormone production, steroid receptor expression in the brain regions governing these traits, neuropeptide/protein hormones and their cognate receptors, and/or through epimutations. It remains to be determined though whether BPA, generally considered a “weak estrogen,” results in similar mechanistic disruptions as phthalates with their predominantly anti-androgenic effects. Both chemicals can potentially disrupt all of the above pathways. Further, it is now apparent that both chemicals may lead to pleiotropic disturbances through steroidogenic and non-steroidogenic pathways (Leon-Olea et al., 2014).
EDC-induced parental and social behavioral deficits could affect the general livelihood, social well-being, ability to attract mate(s), reproduction, and likelihood of successfully rearing young. Thus, early exposure of animal and humans to these widely prevalent chemicals may lead to insidious behavioral effects. Moreover, there is ample evidence in animal models that EDCs (and humans in the case of DES) can induce transgenerational effects (Newbold et al., 2000, 2006; Klip et al., 2002; Titus-Ernstoff et al., 2006; Wolstenholme et al., 2012, 2013; Doyle et al., 2013; Manikkam et al., 2013; Schneider et al., 2013; Zhang et al., 2014b). Therefore, these chemicals might already be exhuming a toll on the social lives of unborn generations. While a call to action to legislate further these chemicals seems a reasonable course of action based on the existing data and precautionary principle, identifying the underpinning mechanisms leading to these behavioral disturbances might provide the incentive for policymakers to act. Long-term studies examining for linkages in parental deficiencies and transgenerational effects in human populations exposed to these EDCs may regrettably be needed before the production of such chemicals is minimized or outright banned.
Conflict of interest statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The studies were supported by NIH Grant 5R21ES023150-02 (to C.S.R.).
References
- Adewale H. B., Todd K. L., Mickens J. A., Patisaul H. B. (2011). The impact of neonatal bisphenol–a exposure on sexually dimorphic hypothalamic nuclei in the female rat. Neurotoxicology 32, 38–49 10.1016/j.neuro.2010.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akingbemi B. T., Ge R., Klinefelter G. R., Zirkin B. R., Hardy M. P. (2004a). Phthalate-induced Leydig cell hyperplasia is associated with multiple endocrine disturbances. Proc. Natl. Acad. Sci. U.S.A. 101, 775–780. 10.1073/pnas.0305977101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akingbemi B. T., Sottas C. M., Koulova A. I., Klinefelter G. R., Hardy M. P. (2004b). Inhibition of testicular steroidogenesis by the xenoestrogen bisphenol A is associated with reduced pituitary luteinizing hormone secretion and decreased steroidogenic enzyme gene expression in rat Leydig cells. Endocrinology 145, 592–603. 10.1210/en.2003-1174 [DOI] [PubMed] [Google Scholar]
- Akingbemi B. T., Youker R. T., Sottas C. M., Ge R., Katz E., Klinefelter G. R., et al. (2001). Modulation of rat Leydig cell steroidogenic function by di(2-ethylhexyl)phthalate. Biol. Reprod. 65, 1252–1259. 10.1095/biolreprod65.4.1252 [DOI] [PubMed] [Google Scholar]
- Arnold A. P., Breedlove S. M. (1985). Organizational and activational effects of sex steroids on brain and behavior: a reanalysis. Horm. Behav. 19, 469–498. 10.1016/0018-506X(85)90042-X [DOI] [PubMed] [Google Scholar]
- Avissar-Whiting M., Veiga K. R., Uhl K. M., Maccani M. A., Gagne L. A., Moen E. L., et al. (2010). Bisphenol A exposure leads to specific microRNA alterations in placental cells. Reprod. Toxicol. 29, 401–406. 10.1016/j.reprotox.2010.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babb J. A., Carini L. M., Spears S. L., Nephew B. C. (2014). Transgenerational effects of social stress on social behavior, corticosterone, oxytocin, and prolactin in rats. Horm. Behav. 65, 386–393. 10.1016/j.yhbeh.2014.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales K. L., Kim A. J., Lewis-Reese A. D., Sue Carter C. (2004). Both oxytocin and vasopressin may influence alloparental behavior in male prairie voles. Horm. Behav. 45, 354–361. 10.1016/j.yhbeh.2004.01.004 [DOI] [PubMed] [Google Scholar]
- Benarroch E. E. (2013). Oxytocin and vasopressin: social neuropeptides with complex neuromodulatory functions. Neurology 80, 1521–1528. 10.1212/WNL.0b013e31828cfb15 [DOI] [PubMed] [Google Scholar]
- Betz A., Jayatilaka S., Joshi J., Ramanan S., Debartolo D., Pylypiw H., et al. (2013). Chronic exposure to benzyl butyl phthalate (BBP) alters social interaction and fear conditioning in male adult rats: alterations in amygdalar MeCP2, ERK1/2 and ERalpha. Neuro Endocrinol. Lett. 34, 347–358. [PubMed] [Google Scholar]
- Beverly B. E., Lambright C. S., Furr J. R., Sampson H., Wilson V. S., McIntyre B. S., et al. (2014). Simvastatin and dipentyl phthalate lower ex vivo testicular testosterone production and exhibit additive effects on testicular testosterone and gene expression via distinct mechanistic pathways in the fetal rat. Toxicol. Sci. 141, 524–537. 10.1093/toxsci/kfu149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botelho G. G., Golin M., Bufalo A. C., Morais R. N., Dalsenter P. R., Martino-Andrade A. J. (2009). Reproductive effects of di(2-ethylhexyl)phthalate in immature male rats and its relation to cholesterol, testosterone, and thyroxin levels. Arch. Environ. Contam. Toxicol. 57, 777–784. 10.1007/s00244-009-9317-8 [DOI] [PubMed] [Google Scholar]
- Boudalia S., Berges R., Chabanet C., Folia M., Decocq L., Pasquis B., et al. (2014). A multi-generational study on low-dose BPA exposure in Wistar rats: effects on maternal behavior, flavor intake and development. Neurotoxicol. Teratol. 41, 16–26. 10.1016/j.ntt.2013.11.002 [DOI] [PubMed] [Google Scholar]
- Braun J. M., Yolton K., Dietrich K. N., Hornung R., Ye X., Calafat A. M., et al. (2009). Prenatal bisphenol A exposure and early childhood behavior. Environ. Health Perspect. 117, 1945–1952. 10.1289/ehp.0900979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun K., Champagne F. A. (2014). Paternal influences on offspring development: behavioural and epigenetic pathways. J. Neuroendocrinol. 26, 697–706. 10.1111/jne.12174 [DOI] [PubMed] [Google Scholar]
- Bredy T. W., Brown R. E., Meaney M. J. (2007). Effect of resource availability on biparental care, and offspring neural and behavioral development in the California mouse (Peromyscus californicus). Eur. J. Neurosci. 25, 567–575. 10.1111/j.1460-9568.2006.05266.x [DOI] [PubMed] [Google Scholar]
- Cao J., Joyner L., Mickens J. A., Leyrer S. M., Patisaul H. B. (2014). Sex-specific Esr2 mRNA expression in the rat hypothalamus and amygdala is altered by neonatal bisphenol A exposure. Reproduction 147, 537–554. 10.1530/REP-13-0501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J., Mickens J. A., McCaffrey K. A., Leyrer S. M., Patisaul H. B. (2012). Neonatal Bisphenol A exposure alters sexually dimorphic gene expression in the postnatal rat hypothalamus. Neurotoxicology 33, 23–36. 10.1016/j.neuro.2011.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J., Rebuli M. E., Rogers J., Todd K. L., Leyrer S. M., Ferguson S. A., et al. (2013). Prenatal bisphenol A exposure alters sex-specific estrogen receptor expression in the neonatal rat hypothalamus and amygdala. Toxicol. Sci. 133, 157–173 10.1093/toxsci/kft035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson A. A., Russell A. F., Young A. J., Jordan N. R., McNeilly A. S., Parlow A. F., et al. (2006). Elevated prolactin levels immediately precede decisions to babysit by male meerkat helpers. Horm. Behav. 50, 94–100. 10.1016/j.yhbeh.2006.01.009 [DOI] [PubMed] [Google Scholar]
- Ceccarelli I., Della Seta D., Fiorenzani P., Farabollini F., Aloisi A. M. (2007). Estrogenic chemicals at puberty change ERalpha in the hypothalamus of male and female rats. Neurotoxicol. Teratol. 29, 108–115. 10.1016/j.ntt.2006.10.011 [DOI] [PubMed] [Google Scholar]
- Champagne F. A., Weaver I. C., Diorio J., Dymov S., Szyf M., Meaney M. J. (2006). Maternal care associated with methylation of the estrogen receptor-alpha1b promoter and estrogen receptor-alpha expression in the medial preoptic area of female offspring. Endocrinology 147, 2909–2915 10.1210/en.2005-1119 [DOI] [PubMed] [Google Scholar]
- Chauvigne F., Plummer S., Lesne L., Cravedi J. P., Dejucq-Rainsford N., Fostier A., et al. (2011). Mono-(2-ethylhexyl) phthalate directly alters the expression of Leydig cell genes and CYP17 lyase activity in cultured rat fetal testis. PLoS ONE 6:e27172 10.1371/journal.pone.0027172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F., Zhou L., Bai Y., Zhou R., Chen L. (2014). Sex differences in the adult HPA axis and affective behaviors are altered by perinatal exposure to a low dose of bisphenol A. Brain Res. 1571, 12–24. 10.1016/j.brainres.2014.05.010 [DOI] [PubMed] [Google Scholar]
- Clutton-Brock T. H. (1989). Mammalian mating systems. Proc. R. Soc. Lond. B Biol. Sci. 236, 339–372. 10.1098/rspb.1989.0027 [DOI] [PubMed] [Google Scholar]
- Crews D., Gore A. C., Hsu T. S., Dangleben N. L., Spinetta M., Schallert T., et al. (2007). Transgenerational epigenetic imprints on mate preference. Proc. Natl. Acad. Sci. U.S.A. 104, 5942–5946. 10.1073/pnas.0610410104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings J. A., Nunez A. A., Clemens L. G. (2005). A cross-fostering analysis of the effects of PCB 77 on the maternal behavior of rats. Physiol. Behav. 85, 83–91. 10.1016/j.physbeh.2005.04.001 [DOI] [PubMed] [Google Scholar]
- D'Cruz S. C., Jubendradass R., Jayakanthan M., Rani S. J., Mathur P. P. (2012). Bisphenol A impairs insulin signaling and glucose homeostasis and decreases steroidogenesis in rat testis: an in vivo and in silico study. Food Chem. Toxicol. 50, 1124–1133. 10.1016/j.fct.2011.11.041 [DOI] [PubMed] [Google Scholar]
- Delclos K. B., Camacho L., Lewis S. M., Vanlandingham M. M., Latendresse J. R., Olson G. R., et al. (2014). Toxicity evaluation of bisphenol A administered by gavage to Sprague Dawley rats from gestation day 6 through postnatal day 90. Toxicol. Sci. 139, 174–197. 10.1093/toxsci/kfu022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Seta D., Minder I., Dessi-Fulgheri F., Farabollini F. (2005). Bisphenol-A exposure during pregnancy and lactation affects maternal behavior in rats. Brain Res. Bull. 65, 255–260. 10.1016/j.brainresbull.2004.11.017 [DOI] [PubMed] [Google Scholar]
- Desdoits-Lethimonier C., Albert O., Le Bizec B., Perdu E., Zalko D., Courant F., et al. (2012). Human testis steroidogenesis is inhibited by phthalates. Hum. Reprod. 27, 1451–1459. 10.1093/humrep/des069 [DOI] [PubMed] [Google Scholar]
- Dessi-Fulgheri F., Porrini S., Farabollini F. (2002). Effects of perinatal exposure to bisphenol A on play behavior of female and male juvenile rats. Environ. Health Perspect. 110(Suppl. 3), 403–407. 10.1289/ehp.02110s3403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty L. F., Bromer J. G., Zhou Y., Aldad T. S., Taylor H. S. (2010). In utero exposure to diethylstilbestrol (DES) or bisphenol-A (BPA) increases EZH2 expression in the mammary gland: an epigenetic mechanism linking endocrine disruptors to breast cancer. Horm. Cancer 1, 146–155 10.1007/s12672-010-0015-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle T. J., Bowman J. L., Windell V. L., McLean D. J., Kim K. H. (2013). Transgenerational effects of di-(2-ethylhexyl) phthalate on testicular germ cell associations and spermatogonial stem cells in mice. Biol. Reprod. 88, 112. 10.1095/biolreprod.112.106104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulac C., O'connell L. A., Wu Z. (2014). Neural control of maternal and paternal behaviors. Science 345, 765–770. 10.1126/science.1253291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engell M. D., Godwin J., Young L. J., Vandenbergh J. G. (2006). Perinatal exposure to endocrine disrupting compounds alters behavior and brain in the female pine vole. Neurotoxicol. Teratol. 28, 103–110. 10.1016/j.ntt.2005.10.002 [DOI] [PubMed] [Google Scholar]
- Environment Canada. (2008). Screening Assessment for the Challenge Phenol, 4,4′ -(1-methylethylidene)bis-(Bisphenol A) Chemical Abstracts Service Registry Number 80-05-7. M.O.T.E.A.O. Health.
- Evans S. F., Kobrosly R. W., Barrett E. S., Thurston S. W., Calafat A. M., Weiss B., et al. (2014). Prenatal Bisphenol A exposure and maternally reported behavior in boys and girls. Neurotoxicology 45, 91–99. 10.1016/j.neuro.2014.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goloubkova T., Ribeiro M. F., Rodrigues L. P., Cecconello A. L., Spritzer P. M. (2000). Effects of xenoestrogen bisphenol A on uterine and pituitary weight, serum prolactin levels and immunoreactive prolactin cells in ovariectomized Wistar rats. Arch. Toxicol. 74, 92–98. 10.1007/s002040050658 [DOI] [PubMed] [Google Scholar]
- Gordon I., Zagoory-Sharon O., Leckman J. F., Feldman R. (2010). Prolactin, Oxytocin, and the development of paternal behavior across the first six months of fatherhood. Horm. Behav. 58, 513–518. 10.1016/j.yhbeh.2010.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandviewresearch. (2014). Global Bisphenol A (BPA) Market by Appliation (Appliances, Automotive, Consumer, Construction, Electrical & Electronics) Expected to Reach USD 20.03 Billion by 2020. Available online at: http://www.digitaljournal.com/pr/2009287 (Accessed July 24, 2014).
- Grasselli F., Baratta L., Baioni L., Bussolati S., Ramoni R., Grolli S., et al. (2010). Bisphenol A disrupts granulosa cell function. Domest. Anim. Endocrinol. 39, 34–39. 10.1016/j.domaniend.2010.01.004 [DOI] [PubMed] [Google Scholar]
- Gubernick D. J., Nelson R. J. (1989). Prolactin and paternal behavior in the biparental California mouse, Peromyscus californicus. Horm. Behav. 23, 203–210. 10.1016/0018-506X(89)90061-5 [DOI] [PubMed] [Google Scholar]
- Guida N., Laudati G., Galgani M., Santopaolo M., Montuori P., Triassi M., et al. (2014). Histone deacetylase 4 promotes ubiquitin-dependent proteasomal degradation of Sp3 in SH-SY5Y cells treated with di(2-ethylhexyl)phthalate (DEHP), determining neuronal death. Toxicol. Appl. Pharmacol. 280, 190–198. 10.1016/j.taap.2014.07.014 [DOI] [PubMed] [Google Scholar]
- Hao Y., Huang W., Nielsen D. A., Kosten T. A. (2011). Litter gender composition and sex affect maternal behavior and DNA methylation levels of the oprm1 gene in rat offspring. Front. Psychiatry 2:21 10.3389/fpsyt.2011.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horstman K. A., Naciff J. M., Overmann G. J., Foertsch L. M., Richardson B. D., Daston G. P. (2012). Effects of transplacental 17-alpha-ethynyl estradiol or bisphenol A on the developmental profile of steroidogenic acute regulatory protein in the rat testis. Birth Defects Res. B Dev. Reprod. Toxicol. 95, 318–325. 10.1002/bdrb.21020 [DOI] [PubMed] [Google Scholar]
- Howdeshell K. L. (2002). A model of the development of the brain as a construct of the thyroid system. Environ. Health Perspect. 110(Suppl. 3), 337–348. 10.1289/ehp.02110s3337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang Y. J., Park H. R., Kim T. H., Yang W. J., Lee J. J., Choi S. Y., et al. (2012). High dose bisphenol A impairs hippocampal neurogenesis in female mice across generations. Toxicology 296, 73–82. 10.1016/j.tox.2012.03.007 [DOI] [PubMed] [Google Scholar]
- Jasarevic E., Sieli P. T., Twellman E. E., Welsh T. H., Jr., Schachtman T. R., Roberts R. M., et al. (2011). Disruption of adult expression of sexually selected traits by developmental exposure to bisphenol A. Proc. Natl. Acad. Sci. U.S.A. 108, 11715–11720. 10.1073/pnas.1107958108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B. A., Shimell J. J., Watson N. V. (2011). Pre- and postnatal bisphenol A treatment results in persistent deficits in the sexual behavior of male rats, but not female rats, in adulthood. Horm. Behav. 59, 246–251. 10.1016/j.yhbeh.2010.12.006 [DOI] [PubMed] [Google Scholar]
- Kaplan L. A., Nabel M., van Cleef-Toedt K., Proffitt A. R., Pylypiw H. M., Jr. (2013). Impact of benzyl butyl phthalate on shoaling behavior in Fundulus heteroclitus (mummichog) populations. Mar. Environ. Res. 86, 70–75. 10.1016/j.marenvres.2013.02.014 [DOI] [PubMed] [Google Scholar]
- Kendziorski J. A., Kendig E. L., Gear R. B., Belcher S. M. (2012). Strain specific induction of pyometra and differences in immune responsiveness in mice exposed to 17alpha-ethinyl estradiol or the endocrine disrupting chemical bisphenol A. Reprod. Toxicol. 34, 22–30 10.1016/j.reprotox.2012.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klip H., Verloop J., van Gool J. D., Koster M. E., Burger C. W., van Leeuwen F. E. (2002). Hypospadias in sons of women exposed to diethylstilbestrol in utero: a cohort study. Lancet 359, 1102–1107. 10.1016/S0140-6736(02)08152-7 [DOI] [PubMed] [Google Scholar]
- Kobrosly R. W., Evans S., Miodovnik A., Barrett E. S., Thurston S. W., Calafat A. M., et al. (2014). Prenatal phthalate exposures and neurobehavioral development scores in boys and girls at 6-10 years of age. Environ. Health Perspect. 122, 521–528. 10.1289/ehp.1307063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konkle A. T., McCarthy M. M. (2011). Developmental time course of estradiol, testosterone, and dihydrotestosterone levels in discrete regions of male and female rat brain. Endocrinology 152, 223–235 10.1210/en.2010-0607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundakovic M., Gudsnuk K., Franks B., Madrid J., Miller R. L., Perera F. P., et al. (2013). Sex-specific epigenetic disruption and behavioral changes following low-dose in utero bisphenol A exposure. Proc. Natl. Acad. Sci. U.S.A. 110, 9956–9961 10.1073/pnas.1214056110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundakovic M., Gudsnuk K., Herbstman J. B., Tang D., Perera F. P., Champagne F. A. (2014). DNA methylation of BDNF as a biomarker of early-life adversity. Proc. Natl. Acad. Sci. U.S.A. [Epub ahead of print]. 10.1073/pnas.1408355111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert K. G., Franssen C. L., Bardi M., Hampton J. E., Hainley L., Karsner S., et al. (2011). Characteristic neurobiological patterns differentiate paternal responsiveness in two Peromyscus species. Brain Behav. Evol. 77, 159–175. 10.1159/000326054 [DOI] [PubMed] [Google Scholar]
- Latini G., De Felice C., Presta G., Del Vecchio A., Paris I., Ruggieri F., et al. (2003). In utero exposure to di-(2-ethylhexyl)phthalate and duration of human pregnancy. Environ. Health Perspect. 111, 1783–1785. 10.1289/ehp.6202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H. C., Yamanouchi K., Nishihara M. (2006). Effects of perinatal exposure to phthalate/adipate esters on hypothalamic gene expression and sexual behavior in rats. J. Reprod. Dev. 52, 343–352. 10.1262/jrd.17096 [DOI] [PubMed] [Google Scholar]
- Lee K. Y., Shibutani M., Takagi H., Kato N., Takigami S., Uneyama C., et al. (2004). Diverse developmental toxicity of di-n-butyl phthalate in both sexes of rat offspring after maternal exposure during the period from late gestation through lactation. Toxicology 203, 221–238. 10.1016/j.tox.2004.06.013 [DOI] [PubMed] [Google Scholar]
- Lee S. H., Kang S. M., Choi M. H., Lee J., Park M. J., Kim S. H., et al. (2014). Changes in steroid metabolism among girls with precocious puberty may not be associated with urinary levels of bisphenol A. Reprod. Toxicol. 44, 1–6 10.1016/j.reprotox.2013.03.008 [DOI] [PubMed] [Google Scholar]
- Leon-Olea M., Martyniuk C. J., Orlando E. F., Ottinger M. A., Rosenfeld C. S., Wolstenholme J. T., et al. (2014). Current concepts in neuroendocrine disruption. Gen. Comp. Endocrinol. 203, 158–173. 10.1016/j.ygcen.2014.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Dai J., Zhang L., Zhang J., Zhang Z., Chen B. (2011). An association of elevated serum prolactin with phthalate exposure in adult men. Biomed. Environ. Sci. 24, 31–39. 10.3967/0895-3988 [DOI] [PubMed] [Google Scholar]
- Lieberwirth C., Wang Z. (2014). Social bonding: regulation by neuropeptides. Front. Neurosci. 8:171. 10.3389/fnins.2014.00171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien Y. J., Ku H. Y., Su P. H., Chen S. J., Chen H. Y., Liao P. C., et al. (2014). Prenatal exposure to phthalate esters and behavioral syndromes in children at eight years of age: Taiwan maternal and infant cohort study. Environ. Health Perspect. 123, 95–100 10.1289/ehp.1307154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Qin F., Wang H., Wu T., Zhang Y., Zheng Y., et al. (2012). Effects of 17alpha-ethinylestradiol and bisphenol A on steroidogenic messenger ribonucleic acid levels in the rare minnow gonads. Aquat. Toxicol. 122–123, 19–27. 10.1016/j.aquatox.2012.05.010 [DOI] [PubMed] [Google Scholar]
- Mahoney M. M., Padmanabhan V. (2010). Developmental programming: impact of fetal exposure to endocrine-disrupting chemicals on gonadotropin-releasing hormone and estrogen receptor mRNA in sheep hypothalamus. Toxicol. Appl. Pharmacol. 247, 98–104. 10.1016/j.taap.2010.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manikkam M., Tracey R., Guerrero-Bosagna C., Skinner M. K. (2013). Plastics derived endocrine disruptors (BPA, DEHP and DBP) induce epigenetic transgenerational inheritance of obesity, reproductive disease and sperm epimutations. PLoS ONE 8:e55387. 10.1371/journal.pone.0055387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Arguelles D., Papadopoulos V. (2015). Identification of hot spots of DNA methylation in the adult male adrenal in response to in utero exposure to the ubiquitous endocrine disruptor plasticizer di-(2-ethylhexyl) phthalate. Endocrinology 156, 124–133. 10.1210/en.2014-1436 [DOI] [PubMed] [Google Scholar]
- McGhee K. E., Bell A. M. (2014). Paternal care in a fish: epigenetics and fitness enhancing effects on offspring anxiety. Proc. Biol. Sci. 281, 20141146. 10.1098/rspb.2014.1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker J. D., Calafat A. M., Hauser R. (2009). Urinary metabolites of di(2-ethylhexyl) phthalate are associated with decreased steroid hormone levels in adult men. J. Androl. 30, 287–297. 10.2164/jandrol.108.006403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miodovnik A., Engel S. M., Zhu C., Ye X., Soorya L. V., Silva M. J., et al. (2011). Endocrine disruptors and childhood social impairment. Neurotoxicology 32, 261–267. 10.1016/j.neuro.2010.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monje L., Varayoud J., Luque E. H., Ramos J. G. (2007). Neonatal exposure to bisphenol A modifies the abundance of estrogen receptor alpha transcripts with alternative 5′-untranslated regions in the female rat preoptic area. J. Endocrinol. 194, 201–212. 10.1677/JOE-07-0014 [DOI] [PubMed] [Google Scholar]
- Monje L., Varayoud J., Munoz-De-Toro M., Luque E. H., Ramos J. G. (2009). Neonatal exposure to bisphenol A alters estrogen-dependent mechanisms governing sexual behavior in the adult female rat. Reprod. Toxicol. 28, 435–442. 10.1016/j.reprotox.2009.06.012 [DOI] [PubMed] [Google Scholar]
- Morris J. A., Jordan C. L., Breedlove S. M. (2004). Sexual differentiation of the vertebrate nervous system. Nat. Neurosci. 7, 1034–1039. 10.1038/nn1325 [DOI] [PubMed] [Google Scholar]
- Nakagami A., Negishi T., Kawasaki K., Imai N., Nishida Y., Ihara T., et al. (2009). Alterations in male infant behaviors towards its mother by prenatal exposure to bisphenol A in cynomolgus monkeys (Macaca fascicularis) during early suckling period. Psychoneuroendocrinology 34, 1189–1197. 10.1016/j.psyneuen.2009.03.005 [DOI] [PubMed] [Google Scholar]
- Nanjappa M. K., Simon L., Akingbemi B. T. (2012). The industrial chemical bisphenol A (BPA) interferes with proliferative activity and development of steroidogenic capacity in rat Leydig cells. Biol. Reprod. 86:135. 10.1095/biolreprod.111.095349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbold R. R., Hanson R. B., Jefferson W. N., Bullock B. C., Haseman J., McLachlan J. A. (2000). Proliferative lesions and reproductive tract tumors in male descendants of mice exposed developmentally to diethylstilbestrol. Carcinogenesis 21, 1355–1363. 10.1093/carcin/21.7.1355 [DOI] [PubMed] [Google Scholar]
- Newbold R. R., Padilla-Banks E., Jefferson W. N. (2006). Adverse effects of the model environmental estrogen diethylstilbestrol are transmitted to subsequent generations. Endocrinology 147, S11–S17. 10.1210/en.2005-1164 [DOI] [PubMed] [Google Scholar]
- Palanza P., Howdeshell K. L., Parmigiani S., Vom Saal F. S. (2002). Exposure to a low dose of bisphenol A during fetal life or in adulthood alters maternal behavior in mice. Environ. Health Perspect. 110, 415–422. 10.1289/ehp.02110s3415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panagiotidou E., Zerva S., Mitsiou D. J., Alexis M. N., Kitraki E. (2014). Perinatal exposure to low-dose bisphenol A affects the neuroendocrine stress response in rats. J. Endocrinol. 220, 207–218. 10.1530/JOE-13-0416 [DOI] [PubMed] [Google Scholar]
- Panzica G., Mura E., Pessatti M., Viglietti-Panzica C. (2005). Early embryonic administration of xenoestrogens alters vasotocin system and male sexual behavior of the Japanese quail. Domest. Anim. Endocrinol. 29, 436–445. 10.1016/j.domaniend.2005.02.010 [DOI] [PubMed] [Google Scholar]
- Parker K. J., Lee T. M. (2001). Central vasopressin administration regulates the onset of facultative paternal behavior in microtus pennsylvanicus (meadow voles). Horm. Behav. 39, 285–294. 10.1006/hbeh.2001.1655 [DOI] [PubMed] [Google Scholar]
- Patisaul H. B., Sullivan A. W., Radford M. E., Walker D. M., Adewale H. B., Winnik B., et al. (2012). Anxiogenic effects of developmental bisphenol A exposure are associated with gene expression changes in the juvenile rat amygdala and mitigated by soy. PLoS ONE 7:e43890. 10.1371/journal.pone.0043890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera F., Vishnevetsky J., Herbstman J. B., Calafat A. M., Xiong W., Rauh V., et al. (2012). Prenatal bisphenol A exposure and child behavior in an inner-city cohort. Environ. Health Perspect. 120, 1190–1194. 10.1289/ehp.1104492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peretz J., Flaws J. A. (2013). Bisphenol A down-regulates rate-limiting Cyp11a1 to acutely inhibit steroidogenesis in cultured mouse antral follicles. Toxicol. Appl. Pharmacol. 271, 249–256. 10.1016/j.taap.2013.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peretz J., Gupta R. K., Singh J., Hernandez-Ochoa I., Flaws J. A. (2011). Bisphenol A impairs follicle growth, inhibits steroidogenesis, and downregulates rate-limiting enzymes in the estradiol biosynthesis pathway. Toxicol. Sci. 119, 209–217. 10.1093/toxsci/kfq319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phoenix C. H., Goy R. W., Gerall A. A., Young W. C. (1959). Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology 65, 369–382. 10.1210/endo-65-3-369 [DOI] [PubMed] [Google Scholar]
- Poimenova A., Markaki E., Rahiotis C., Kitraki E. (2010). Corticosterone-regulated actions in the rat brain are affected by perinatal exposure to low dose of bisphenol A. Neuroscience 167, 741–749. 10.1016/j.neuroscience.2010.02.051 [DOI] [PubMed] [Google Scholar]
- Porrini S., Belloni V., Della Seta D., Farabollini F., Giannelli G., Dessi-Fulgheri F. (2005). Early exposure to a low dose of bisphenol A affects socio-sexual behavior of juvenile female rats. Brain Res. Bull. 65, 261–266. 10.1016/j.brainresbull.2004.11.014 [DOI] [PubMed] [Google Scholar]
- Ramos J. G., Varayoud J., Kass L., Rodriguez H., Costabel L., Munoz-De-Toro M., et al. (2003). Bisphenol a induces both transient and permanent histofunctional alterations of the hypothalamic-pituitary-gonadal axis in prenatally exposed male rats. Endocrinology 144, 3206–3215. 10.1210/en.2002-0198 [DOI] [PubMed] [Google Scholar]
- Razzoli M., Valsecchi P., Palanza P. (2005). Chronic exposure to low doses bisphenol A interferes with pair-bonding and exploration in female Mongolian gerbils. Brain Res. Bull. 65, 249–254. 10.1016/j.brainresbull.2004.11.013 [DOI] [PubMed] [Google Scholar]
- Rice D., Barone S., Jr. (2000). Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ. Health Perspect. 108(Suppl. 3), 511–533. 10.1289/ehp.00108s3511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling J. K., Young L. J. (2014). The biology of mammalian parenting and its effect on offspring social development. Science 345, 771–776. 10.1126/science.1252723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld C. S. (2014). Animal models of transgenerational epigenetic effects, in Transgenerational Epigenetics, ed. Tollefsbol T. (London: Elsevier Publications; ), 123–145. [Google Scholar]
- Saaristo M., Craft J. A., Lehtonen K. K., Lindstrom K. (2009). Sand goby (Pomatoschistus minutus) males exposed to an endocrine disrupting chemical fail in nest and mate competition. Horm. Behav. 56, 315–321. 10.1016/j.yhbeh.2009.06.010 [DOI] [PubMed] [Google Scholar]
- Saaristo M., Craft J. A., Lehtonen K. K., Lindstrom K. (2010). An endocrine disrupting chemical changes courtship and parental care in the sand goby. Aquat. Toxicol. 97, 285–292. 10.1016/j.aquatox.2009.12.015 [DOI] [PubMed] [Google Scholar]
- Saillenfait A. M., Sabate J. P., Robert A., Rouiller-Fabre V., Roudot A. C., Moison D., et al. (2013). Dose-dependent alterations in gene expression and testosterone production in fetal rat testis after exposure to di-n-hexyl phthalate. J. Appl. Toxicol. 33, 1027–1035. 10.1002/jat.2896 [DOI] [PubMed] [Google Scholar]
- Savchuk I., Soder O., Svechnikov K. (2013). Mouse leydig cells with different androgen production potential are resistant to estrogenic stimuli but responsive to bisphenol a which attenuates testosterone metabolism. PLoS ONE 8:e71722. 10.1371/journal.pone.0071722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider S., Marxfeld H., Groters S., Buesen R., van Ravenzwaay B. (2013). Vinclozolin–no transgenerational inheritance of anti-androgenic effects after maternal exposure during organogenesis via the intraperitoneal route. Reprod. Toxicol. 37, 6–14. 10.1016/j.reprotox.2012.12.003 [DOI] [PubMed] [Google Scholar]
- Spearow J. L., Doemeny P., Sera R., Leffler R., Barkley M. (1999). Genetic variation in susceptibility to endocrine disruption by estrogen in mice. Science 285, 1259–1261. 10.1126/science.285.5431.1259 [DOI] [PubMed] [Google Scholar]
- Spearow J. L., O'Henley P., Doemeny P., Sera R., Leffler R., Sofos T., et al. (2001). Genetic variation in physiological sensitivity to estrogen in mice. APMIS 109, 356–364. 10.1034/j.1600-0463.2001.090504.x [DOI] [PubMed] [Google Scholar]
- Steinberg R. M., Juenger T. E., Gore A. C. (2007). The effects of prenatal PCBs on adult female paced mating reproductive behaviors in rats. Horm. Behav. 51, 364–372. 10.1016/j.yhbeh.2006.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz R., Brown N. G., Allen D. L., Bigsby R. M., Ben-Jonathan N. (1997). The environmental estrogen bisphenol A stimulates prolactin release in vitro and in vivo. Endocrinology 138, 1780–1786. [DOI] [PubMed] [Google Scholar]
- Stoker C., Beldomenico P. M., Bosquiazzo V. L., Zayas M. A., Rey F., Rodriguez H., et al. (2008). Developmental exposure to endocrine disruptor chemicals alters follicular dynamics and steroid levels in Caiman latirostris. Gen. Comp. Endocrinol. 156, 603–612. 10.1016/j.ygcen.2008.02.011 [DOI] [PubMed] [Google Scholar]
- Sullivan A. W., Beach E. C., Stetzik L. A., Perry A., D'Addezio A. S., Cushing B. S., et al. (2014). A novel model for neuroendocrine toxicology: neurobehavioral effects of BPA exposure in a prosocial species, the prairie vole (Microtus ochrogaster). Endocrinology 155, 3867–3881. 10.1210/en.2014-1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supornsilchai V., Soder O., Svechnikov K. (2007). Stimulation of the pituitary-adrenal axis and of adrenocortical steroidogenesis ex vivo by administration of di-2-ethylhexyl phthalate to prepubertal male rats. J. Endocrinol. 192, 33–39. 10.1677/JOE-06-0004 [DOI] [PubMed] [Google Scholar]
- Svechnikov K., Svechnikova I., Soder O. (2008). Inhibitory effects of mono-ethylhexyl phthalate on steroidogenesis in immature and adult rat Leydig cells in vitro. Reprod. Toxicol. 25, 485–490. 10.1016/j.reprotox.2008.05.057 [DOI] [PubMed] [Google Scholar]
- Tang W. Y., Morey L. M., Cheung Y. Y., Birch L., Prins G. S., Ho S. M. (2012). Neonatal exposure to estradiol/bisphenol A alters promoter methylation and expression of Nsbp1 and Hpcal1 genes and transcriptional programs of Dnmt3a/b and Mbd2/4 in the rat prostate gland throughout life. Endocrinology 153, 42–55. 10.1210/en.2011-1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilghman S. L., Bratton M. R., Segar H. C., Martin E. C., Rhodes L. V., Li M., et al. (2012). Endocrine disruptor regulation of microRNA expression in breast carcinoma cells. PLoS ONE 7:e32754. 10.1371/journal.pone.0032754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titus-Ernstoff L., Troisi R., Hatch E. E., Wise L. A., Palmer J., Hyer M., et al. (2006). Menstrual and reproductive characteristics of women whose mothers were exposed in utero to diethylstilbestrol (DES). Int. J. Epidemiol. 35, 862–868. 10.1093/ije/dyl106 [DOI] [PubMed] [Google Scholar]
- Vandenberg L. N., Ehrlich S., Belcher S. M., Ben-Jonathan N., Dolinoy D. C., Hugo E. S., et al. (2013). Low dose effects of bisphenol A: an integrated review of in vitro, laboratory animal and epidemiology studies. Endocr. Disruption 1, E1–E20 10.4161/endo.26490 [DOI] [Google Scholar]
- Veiga-Lopez A., Luense L. J., Christenson L. K., Padmanabhan V. (2013). Developmental programming: gestational bisphenol-A treatment alters trajectory of fetal ovarian gene expression. Endocrinology 154, 1873–1884. 10.1210/en.2012-2129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker S. C., McGlone F. P. (2013). The social brain: neurobiological basis of affiliative behaviours and psychological well-being. Neuropeptides 47, 379–393. 10.1016/j.npep.2013.10.008 [DOI] [PubMed] [Google Scholar]
- Ward J. L., Blum M. J. (2012). Exposure to an environmental estrogen breaks down sexual isolation between native and invasive species. Evol. Appl. 5, 901–912. 10.1111/j.1752-4571.2012.00283.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warita K., Mitsuhashi T., Ohta K., Suzuki S., Hoshi N., Miki T., et al. (2013). Gene expression of epigenetic regulatory factors related to primary silencing mechanism is less susceptible to lower doses of bisphenol A in embryonic hypothalamic cells. J. Toxicol. Sci. 38, 285–289. 10.2131/jts.38.285 [DOI] [PubMed] [Google Scholar]
- Watson J., Adkins-Regan E. (1989). Activation of sexual behavior by implantation of testosterone propionate and estradiol benzoate into the preoptic area of the male Japanese quail (Coturnix japonica). Horm. Behav. 23, 251–268. 10.1016/0018-506X(89)90065-2 [DOI] [PubMed] [Google Scholar]
- Weaver I. C. (2007). Epigenetic programming by maternal behavior and pharmacological intervention. Nature versus nurture: let's call the whole thing off. Epigenetics 2, 22–28 10.4161/epi.2.1.3881 [DOI] [PubMed] [Google Scholar]
- Weaver I. C., Meaney M. J., Szyf M. (2006). Maternal care effects on the hippocampal transcriptome and anxiety-mediated behaviors in the offspring that are reversible in adulthood. Proc. Natl. Acad. Sci. U.S.A. 103, 3480–3485. 10.1073/pnas.0507526103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wibe A. E., Billing A., Rosenqvist G., Jenssen B. M. (2002). Butyl benzyl phthalate affects shoaling behavior and bottom-dwelling behavior in threespine stickleback. Environ. Res. 89, 180–187. 10.1006/enrs.2002.4360 [DOI] [PubMed] [Google Scholar]
- Williams S. A., Jasarevic E., Vandas G. M., Warzak D. A., Geary D. C., Ellersieck M. R., et al. (2013). Effects of developmental bisphenol A exposure on reproductive-related behaviors in California mice (Peromyscus californicus): a monogamous animal model. PLoS ONE 8:e55698. 10.1371/journal.pone.0055698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme J. T., Edwards M., Shetty S. R., Gatewood J. D., Taylor J. A., Rissman E. F., et al. (2012). Gestational exposure to bisphenol a produces transgenerational changes in behaviors and gene expression. Endocrinology 153, 3828–3838. 10.1210/en.2012-1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme J. T., Goldsby J. A., Rissman E. F. (2013). Transgenerational effects of prenatal bisphenol A on social recognition. Horm. Behav. 64, 833–839. 10.1016/j.yhbeh.2013.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme J. T., Rissman E. F., Connelly J. J. (2011a). The role of bisphenol A in shaping the brain, epigenome and behavior. Horm. Behav. 59, 296–305. 10.1016/j.yhbeh.2010.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme J. T., Taylor J. A., Shetty S. R., Edwards M., Connelly J. J., Rissman E. F. (2011b). Gestational exposure to low dose bisphenol A alters social behavior in juvenile mice. PLoS ONE 6:e25448. 10.1371/journal.pone.0025448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wudarczyk O. A., Earp B. D., Guastella A., Savulescu J. (2013). Could intranasal oxytocin be used to enhance relationships? Research imperatives, clinical policy, and ethical considerations. Curr. Opin. Psychiatry 26, 474–484. 10.1097/YCO.0b013e3283642e10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynne-Edwards K. E., Timonin M. E. (2007). Paternal care in rodents: weakening support for hormonal regulation of the transition to behavioral fatherhood in rodent animal models of biparental care. Horm. Behav. 52, 114–121. 10.1016/j.yhbeh.2007.03.018 [DOI] [PubMed] [Google Scholar]
- Yaoi T., Itoh K., Nakamura K., Ogi H., Fujiwara Y., Fushiki S. (2008). Genome-wide analysis of epigenomic alterations in fetal mouse forebrain after exposure to low doses of bisphenol A. Biochem. Biophys. Res. Commun. 376, 563–567. 10.1016/j.bbrc.2008.09.028 [DOI] [PubMed] [Google Scholar]
- Yeo M., Berglund K., Hanna M., Guo J. U., Kittur J., Torres M. D., et al. (2013). Bisphenol A delays the perinatal chloride shift in cortical neurons by epigenetic effects on the Kcc2 promoter. Proc. Natl. Acad. Sci. U.S.A. 110, 4315–4320. 10.1073/pnas.1300959110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Xu X., Li T., Lu Y., Ruan Q., Lu Y., et al. (2014a). Exposure to bisphenol-A affects fear memory and histone acetylation of the hippocampus in adult mice. Horm. Behav. 65, 106–113. 10.1016/j.yhbeh.2013.12.004 [DOI] [PubMed] [Google Scholar]
- Zhang W. Z., Yong L., Jia X. D., Li N., Fan Y. X. (2013). Combined subchronic toxicity of bisphenol A and dibutyl phthalate on male rats. Biomed. Environ. Sci. 26, 63–69. 10.3967/0895-3988.2013.01.008 [DOI] [PubMed] [Google Scholar]
- Zhang X. F., Zhang T., Han Z., Liu J. C., Liu Y. P., Ma J. Y., et al. (2014b). Transgenerational inheritance of ovarian development deficiency induced by maternal diethylhexyl phthalate exposure. Reprod. Fertil. Dev. [Epub ahead of print]. 10.1071/RD14113 [DOI] [PubMed] [Google Scholar]
- Zhao Y., Shi H. J., Xie C. M., Chen J., Laue H., Zhang Y. H. (2014). Prenatal phthalate exposure, infant growth, and global DNA methylation of human placenta. Environ. Mol. Mutagen. [Epub ahead of print]. 10.1002/em.21916 [DOI] [PubMed] [Google Scholar]
- Zhou R., Chen F., Chang F., Bai Y., Chen L. (2013). Persistent overexpression of DNA methyltransferase 1 attenuating GABAergic inhibition in basolateral amygdala accounts for anxiety in rat offspring exposed perinatally to low-dose bisphenol A. J. Psychiatr. Res. 47, 1535–1544. 10.1016/j.jpsychires.2013.05.013 [DOI] [PubMed] [Google Scholar]