Summary
To ensure proper cell function, intracellular organelles are not randomly distributed within the cell, but polarized and highly constrained by the cytoskeleton and associated adaptor proteins. This relationship between distribution and function was originally found in neurons and epithelial cells; however, recent evidence suggests that it is a more general phenomenon that occurs in many highly specialized cells including the immune T cells. Recent studies reveal that the orchestrated redistribution of organelles is dependent on antigen specific activation and immune-synapse formation of T cells. This review highlights the functional implications of organelle polarization in early T cell activation and examines recent findings on how the immune synapse sets the rhythm of organelle motion and the spread of the activation signal to the nucleus.
Keywords: Immune synapse, microcluster, signalosome, mitochondria, vesicle, microtubule
Overview of the immune synapse
T cell activation starts with an initial wave of signalling that depends on the activation of surface receptors, but subsequent propagation of the signal appears to depend on intracellular compartments, in a sequence driven by the actin and tubulin cytoskeletons [1]. The study of neural synapses highlighted the importance of cell polarity and the specific localization of organelles and clusters of receptors at the pre- and post-synaptic terminals. Despite its transient nature, recent evidence shows that the immune synapse (IS) shares these same neuronal features.
The IS is the interface between a T lymphocyte and an antigen presenting cell (APC). During the formation and stabilization of the IS, membrane microdomains and the cytoskeleton reorganize and promote the segregation of membrane and intracellular signaling proteins within the T cell, such as the T cell receptor (TCR), integrins, tyrosine kinases and scaffold proteins such as Linker for activation of T cells (LAT) (Figure 1 and Glossary). The TCR and associated molecules (TCR signalosomes) form microclusters that move to congregate in the central area (central supramolecular activation cluster; cSMAC) of the IS, whereas adhesion receptors such as integrins reorganize in a surrounding external ring called the peripheral or pSMAC (Figure 2) [2]. In addition, phosphorylation and dephosphorylation, protein-protein interactions and degradation of important molecules such as the TCR-accompanying CD3 complex, tyrosine kinases, phosphatases and adaptor molecules also control receptor activation [3, 4]. The polarization of several organelles occurs during T cell stimulation with important roles, such as the centrosome, Golgi apparatus, mitochondria, endoplasmic reticulum and the multivesicular bodies (MVB) [5-10]. These rearrangements at the IS promote the activation of signaling pathways that propagate to the nucleus. Signals activating nuclear transcription factors in combination with polarity proteins, such as partitioning defective (Par) and Discs large (Dlg)-Scribble (Scrib)-Lethal giant larvae (Lgl) complexes, promote asymmetric division of the activated T cells to allow T cell differentiation and proliferation [11-13].
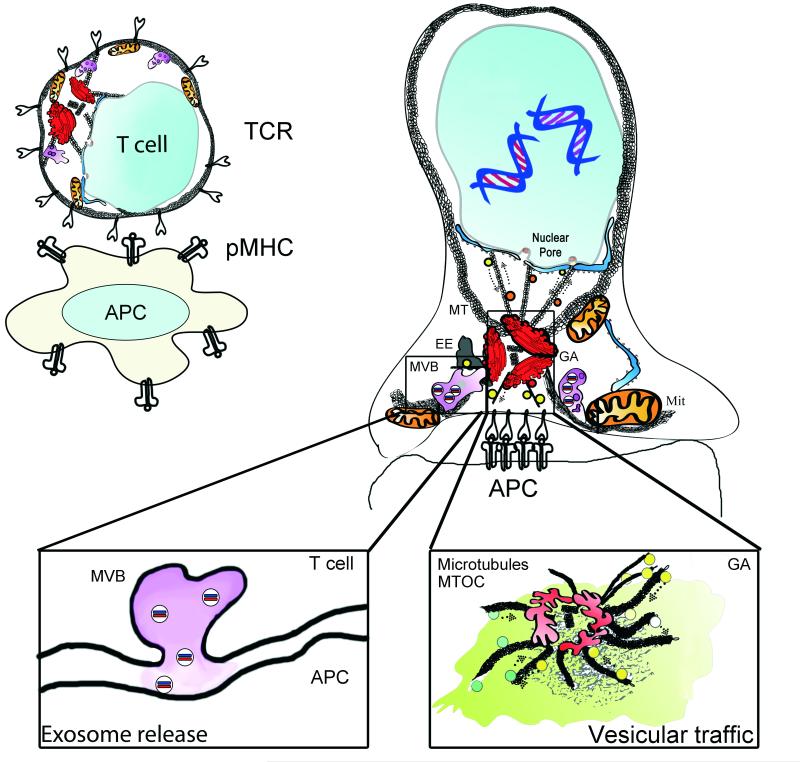
Figure 1. Polarization of organelles at the immune synapse.
The IS forms rapidly upon focal TCR activation. The TCR accumulates at the interface of the T cell and the stimulating surface; an antigen-presenting cell (APC) or a surface coated with stimulating antibodies or recombinant proteins, such as the MHC complex. The centrosome localizes to the IS and brings the associated Golgi Apparatus (GA). The early endosome compartment (EE) regulates recycling of surface receptors. The mitochondrial network (Mit), in association with the endoplasmic reticulum, dynamically localizes to the IS and provides a controlled calcium flux and a focused supply of ATP for energy provision. Nuclear activating molecules enter the nucleus via nuclear pores. They can be transported in vesicles or through direct interaction with molecular motors such as Dynein. Left inset: Multivesicular bodies (MVB) localize to the IS upon centrosome relocalization and have several functions, including the recycling of proteins and the formation of exosomes. MVBs serve as exocytosis compartments at the IS. Right inset: vesicular traffic at the IS. Vesicles coming from the IS are directed to recycling organelles, such as endosomes. While a number of vesicles emerging from the trans-Golgi network facilitate polarized secretion.
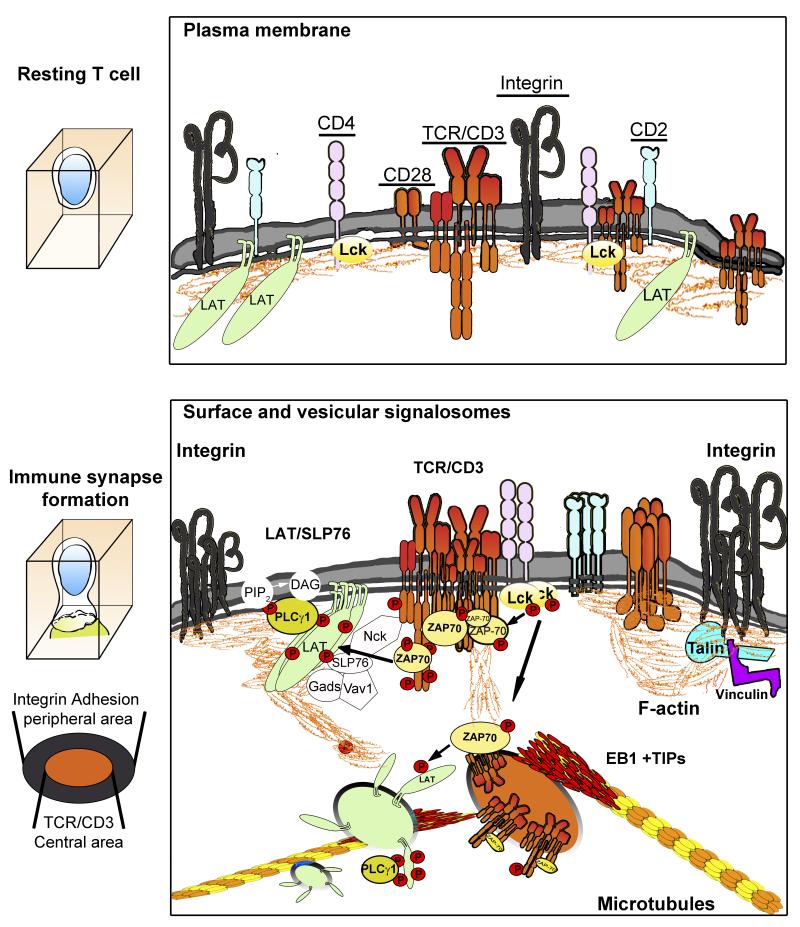
Figure 2. Crosstalk between signals and organelles of resting and activated T cells.
In resting T cells, signaling receptors are evenly distributed throughout the plasma membrane. After the TCR engages an antigen-presenting cell, signaling receptors and molecules form microclusters, composed of different signalosomes, to propagate the signal. Below the plasma membrane, microtubule-dependent, subcortical vesicular trafficking helps to spread signals from the TCR toward the nucleus. The interchange of molecules between CD3, LAT and SLP76 clusters and CD3- and LAT-bearing vesicles can amplify these signaling pathways. The interaction of CD3 and LAT-containing vesicles is another mechanism for signal spread at the IS.
The cross-talk between the T cell receptor, organelle movement to the IS, and the signals leading to nuclear activation offer exciting perspectives to find new targets to regulate T cell activation during immune responses or undesirable immune activation. This review aims to focus on the mechanisms by which receptors, signaling and polarity proteins and organelle polarization may directly influence T cell activation. In addition, we will discuss how the persistence in cell asymmetry imposed by the IS may regulate asymmetric cell division and lymphocyte differentiation during proliferation of T cells, therefore fine-tuning the immune response.
The centrosome marks the way
In neurons, the establishment of polarity depends largely on the growth and stability of microtubules [14], accompanied by the localization of organelles [15, 16]. Centrosome or microtubule-organizing centre (MTOC; Glossary) localization determines this initial polarity during development, establishing the site of Golgi localization and the trafficking of vesicles and mitochondria [16]. These events are paralleled in T cells as early analysis of T cell activation established the direct involvement of the centrosome in the localization of the Golgi apparatus (GA) at the IS (Figure 1) [5, 7, 8, 17-19].
Early upon TCR activation, the centrosome localizes to the IS and rapidly polymerizes α/β tubulin dimers in microtubules [18], which organize a profuse network of microtubules at the T cell-APC contact area [18, 20]. This dynamic network, regulated by microtubule-associated proteins (MAPs) and tubulin post-translational modifications (PTMs; Box 1), provides a platform for intracellular transport and integration of the actin and tubulin cytoskeletons [19]. The reorientation of the Golgi and associated vesicles depends on the correct localization of the centrosome at the IS, thereby favoring polarized secretion of cytokines and microvesicles by CD4+ T effector cells [6, 10] and lytic granules by cytotoxic CD8+ T cells (CTLs) [21]. However, recent evidence also supports a role for microtubule polymerization in IS-directed cytokine secretion of IFN-γ by CD4+ T cells independent of centrosome polarization [22]. Additionally in CD8+ CTLs, suboptimal activation of the TCR can allow centrosomal-independent exocytosis of lytic granules before centrosome translocation [23, 24]. Nonetheless, full activation of T lymphocytes largely depends on centrosome polarization [17].
Box1. Microtubule polymerization and postranslational modifications of the microtubule rail.
Microtubule post-translational modifications (PTMs) including acetylation, tyrosination/detyrosination, poly-glutamylation or glycination, are also important for microtubule function (Figure I). Acetylation of tubulin by alpha-tubulin acetyltransferase (MEC-17, αTAT-1) is the only PTM occurring on the microtubular luminal surface [83], and could control the binding of putative intraluminal proteins identified in cilia microtubule doublets [84]. The role of αTAT-1 seems to be complex because its interaction with microtubules, independent of its enzymatic activity, can promote a major catastrophe ratio, which is in opposition of its classical role as a marker of stable skeletons [85]. Deacetylation of tubulin at Lys 40 is an early event of T cell activation that coincides with centrosome translocation, and depends on the activity of HDAC6, as HDAC6 overexpression prevents centrosome translocation [86]. Sirtuin, a different tubulin deacetylase has not been analysed in the IS formation yet, but can constitute another regulator [87]. Tyrosination and detyrosination of microtubules is dependent on the equilibrium of tyrosine-tubulin ligase (TTL) and carboxypeptidase activities (CpA), which add and remove tyrosine from the C-terminal EEY motif of α–tubulin. Detyrosination controls the rescue/catastrophe ratio by regulating MCAK, a kinesin depolymerizing factor whose interaction is regulated by EEY motifs [88]. Detyrosination of microtubules has been observed during T cell activation, and is promoted by a formin-like protein (IFN2). Knock-down of IFN2 abrogates centrosome translocation, supporting the idea that T cell activation requires a stabilized microtubule network [46].
The state of tyrosination and detyrosination of microtubules can also regulate the binding of microtubule-associated proteins, such as Cap-Gly proteins (CLIP170, p150 dynactin), which can in turn modulate the growth of microtubules and the binding of cargoes. Indeed, this growth and binding are regulated by several proteins, including the EB family of proteins that can contribute large numbers of recruiting C-terminal EEY motifs (Figure I) [87]. EB1 can regulate vesicular trafficking during ciliogenesis, where the centrosome is an important cue for polarity [89]. A similar regulation has also been observed during IS formation [18]. Moreover, the IS is a focus for the localization of intraflagellar transport system (IFT) components , which regulate TCR sorting and bind microtubules to allow transport of vesicles [90].
The binding and gliding of microtubule-based motors is dependent on polyglutamylation (E) of microtubules in vivo. Enzymes of the Tubulin Tyrosine Ligase-Like family (TTLL) regulate this modification. Although not detected in human cells, polyglycilation was initially studied as a regulator of different microtubule-associated proteins (molecular motors and MAPs, Figure I). The mammalian TTLL10 enzyme, which extends monoglycylation produced by TTLL3 and 8, has suffered an evolutionary mutation and has no enzymatic activity in humans [87]. Whether these PTMs are important for T cell activation is unknown. Collectively, microtubule PTMs form part of the regulatory repertoire needed for fine tuning TCR activation.
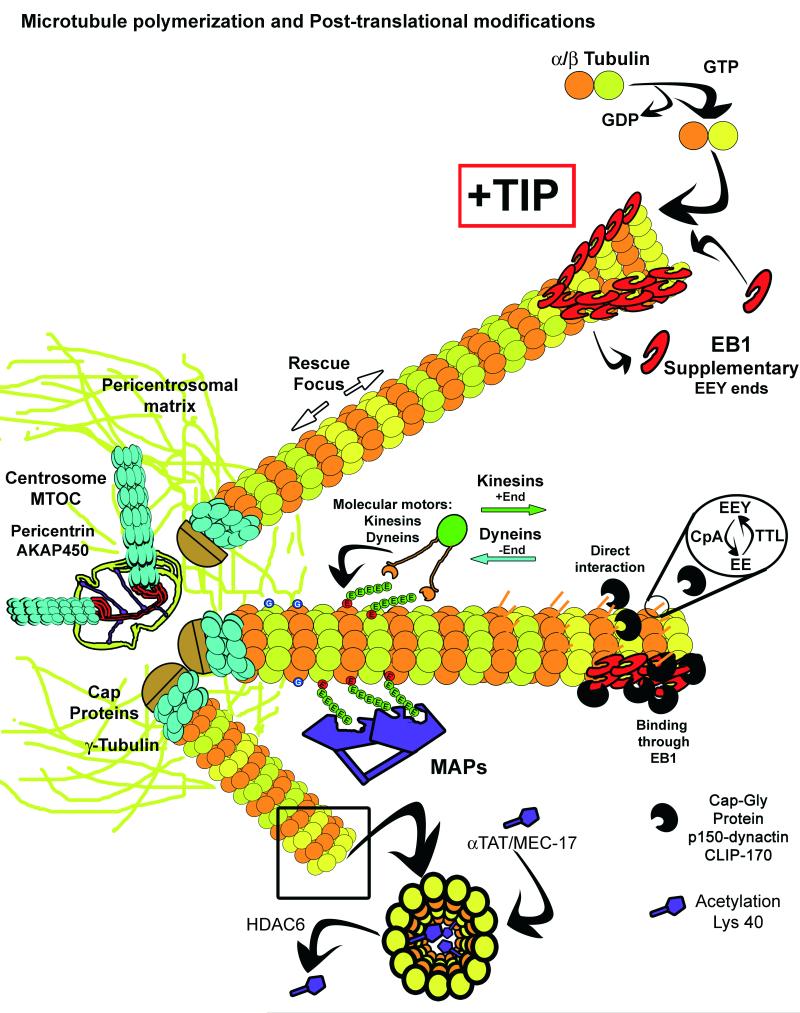
Figure I.
The microtubule network emerges from the centrosome and the pericentrosomal matrix allows the binding of γ-Tubulin Ring Complexes (γTuRC), formed by γ-Tubulin and capping proteins. In association with microtubule plus-end tracking proteins (+TIPS), the γTuRCs provide stable plus-ends that allow microtubules to grow. One such +TIPS, end-binding 1 rotein (EB1), facilitates the incorporation of α/β-Tubulin heterodimers into a sheet formed by the tubulin protofilaments, upon GDP interchange by GTP in the β-Tubulin subunit. EB proteins and proteins containing the TOG domain (XMAP125, CLASP) help fold the sheet into a tubule. The GTP then hydrolyzes into GDP and facilitates the catastrophe events that depolymerize the microtubules. However, “GTP seeds” may remain to serve as a rescue focus in preexisting microtubules.
TCR activation drives centrosome translocation and anti-CD3 antibodies can mimic this translocation. During T cell activation, members of the src family of kinases, Lck and Fyn, phosphorylate the immunoreceptor tyrosine-based activation motifs (ITAM) of the CD3 complex, permitting subsequent binding and activation of zeta-associated protein 70 (ZAP70), a tyrosine kinase that regulates the propagation of the TCR signal (Figure 2) [2]. Lck deficient cells cannot retain the centrosome at the IS, suggesting that Lck may control centrosome docking. Alternatively, Fyn dependent-TCR activation alone may not be enough to reorient the centrosome fully. Furthermore, these cells are defective in cytokine production upon TCR stimulation [25, 26], supporting a role for centrosome translocation in the production and secretion of cytokines. Immediately following TCR activation, localized spikes in the concentration of diacylglycerol (DAG), produced by phospholipase Cγ1 (PLCγ1), relocalize the centrosome to a particular position in the plasma membrane [27]. These DAG-rich membrane areas also act as the focus for localization of the microtubule-driven motor dynein [27]. Dynein/dynactin complex is involved in the translocation of the centrosome to the IS [17]. Dynein may help dock the centrosome by interacting with Fyb/ADAP at the integrin ring of the IS [2]. Additionally, dynein/dynactin disruption prevents correct localization of integrins at the peripheral area of the IS [17]. T cell integrins including leukocyte-function associated 1 (LFA-1) and very late antigen 4 (VLA4) [28], reorganize at the IS to provide an adhesive ring that surrounds the TCR-enriched central area and stabilizes the IS. Disruption of centrosome-localized proteins involved in the integrin signalling complex, such as AKAP9, prevent the localization of the centrosome at the IS and the local activation of integrin LFA-1 [29].
Centrosome polarization at the IS also allows trafficking of the membrane adaptor protein Linker for activation of T cells (LAT) to the IS. LAT is phosphorylated at Y132 by ZAP70, allowing PLCγ1 binding and activation [30]. LAT partitions into two pools, one at the plasma membrane and a second, located in intracellular compartments (Figure 2). Upon phosphorylation, plasma membrane localized LAT redirects the intracellular LAT pool to the nascent IS [30, 31]. The initial TCR signal is amplified after CD3 and LAT signalosomes localize to their corresponding microclusters at the plasma membrane [32], as well as after their recycling and sorting from intracellular stores. Indeed, the synchronized traffic of LAT-enriched vesicles and plasma membrane LAT clusters has been detected by total internal reflexion fluorescence microscopy (TIRFM) and linked to sustained TCR signaling [33]. However, a recent study describes that LAT remains unphosphorylated at pre-existing clusters at the plasma membrane, suggesting T cell activation depends on intracellular traffic [34]. With photoactivated localization microscopy (PALM), LAT microclusters were found surrounded by the adaptor protein SH2 domain-containing leukocyte (SLP76) containing “corrals”, thereby localizing the activation signal to the center [35]. The movement of SLP76 microclusters, which was dependent on polarity protein Dlg1, correlates with the distribution of the microtubular network [1, 19]. SLP76 can also regulate the activity of ZAP70, promoting microcluster formation: LAT recruitment to the IS is not altered by diminished ZAP70 clustering in SLP76-deficient cells [36]. SLP76 also recruits numerous proteins that converge on cytoskeletal regulators Rac/Cdc42, including Nck and Vav, and can therefore promote F-actin modification (Figure 2) [30, 37]. These data suggest a regulated movement of microclusters and cortical vesicles might influence T cell activation through the release of new molecules to microclusters or by the movement of activated molecules from the cell membrane to intracellular compartments.
Vesicular traffic at the IS
Vesicular trafficking at the IS has traditionally been understood in terms of protein sorting and recycling to allow the renewal of plasma membrane molecules important for T cell activation [31, 38]. In resting and activated T cells, CD3 is actively trafficked in vesicles to renew the TCR complex at the cell surface through the recycling of activated and bystander receptors from the plasma membrane [39]. The renewal of TCR/CD3 complexes at the IS is equally important for both T cell activation and for signal inactivation. Both processes depend closely on the cytoskeletal organization at the IS. Below, we discuss various mechanisms to activate and inhibit T cell signalling.
During T cell activation, the central part of the IS, also known as cSMAC, appears devoid of F-actin upon concentration of TCR/CD3 complexes [40], which allows the microtubule-mediated transport, docking and fusion of CD3-bearing vesicles via SNARE proteins, such as VAMP3 in vesicles and syntaxin at the plasma membrane [38]. Recent data indicate that trafficking of LAT-bearing vesicles to the cortical region of the IS is regulated by VAMP7. In contrast to the CD3-bearing vesicles controlled by VAMP3, these LAT-bearing vesicles do not fuse with the plasma membrane, but control LAT phosphorylation upon TCR activation [41]. Contact between microclusters and subcortical vesicles has also been observed in T cell–APC conjugates using optical tweezers [42]. Indeed, analysis of EB1 at the nascent IS by TIRF and confocal microscopy has revealed a role for this protein in the movement of cortical vesicles below CD3 microclusters present at the plasma membrane (Figure 2). Lack of EB1 disrupts the encounter between LAT- and CD3-vesicles at the IS and prevents sustained activation of LAT and PLCγ1 upon TCR stimulation [18]. This finding correlates with the accumulation of highly phosphorylated CD3ζ in intracellular compartments corresponding to Rab7 and Rab11 endosomes, which may result in significant intracellular activation [43]. Rab7 marks late endosomes while Rab11 marks recycling endosomes directed to the pericentrosomal region [44]. The adaptor protein uncoordinated 119 (Unc 119) favors the interaction of Rab11 with the molecular motor Myosin 5B, thereby regulating the movement of Rab11+ endosomes that transport Lck to the IS from the pericentrosomal region [45]. Whether CD3ζ vesicles from the IS correspond to Lck vesicles at the pericentrosomal region and can cycle between the intracellular compartment of signaling molecules and the IS will require further investigation. Future studies should also address whether this traffic relates to MAL-mediated transport of Lck and possibly controlled by the detyrosination of microtubules promoted by IFN2 [46].
In addition, there is intense traffic associated with the shutdown of the T cell signal. Several studies suggest that an intracellular signalling component, which is dependent on vesicular trafficking beneath the IS, regulates cell surface derived TCR signalling. The endocytosis of the TCR has been studied through its post-translational modification by ubiquitination, which was initially considered a marker for intracellular degradation in the proteasome, but is now known to regulate its activity [47]. The central area is a focal point for CD3 endocytosis by the ubiquitin-binding protein tsg101, an ESCRTI complex component [48]. In this regard, endocytosis of TCR/CD3 complexes depends on the GTPase Rab35, which is located at the plasma membrane [49]. In addition, the central IS may also be a site for this clathrin-independent endocytosis since it imports pMHC complexes bound to the TCR/CD3 signalosomes via the GTPases TC21 and RhoG on the actin-cytoskeleton [50]. A similar transendocytic process has been described for the negative regulator CTLA4 and its co-receptors at the APC, CD80 and CD86, which it shares with the costimulatory molecule CD28 [51]. Here, reduced T cell activation primarily occurs by reducing the number of costimulatory CD28 molecules engaged by CD80 or CD86. Moreover, CTLA4-bearing vesicles polarize and group behind the TCR area at the IS, from where their content is released upon CD80 binding, thereby preventing CD28-mediated costimulation [52]. Therefore, protein composition of a stimulated T cell can either be regulated by ubiquitination and proteasome degradation [53, 54], or by uptake of membrane patches from the APC. Future studies should address if these components are sufficient to control the final destiny of TCR/CD3 complexes: will they be directed to recycling or degradation pathways at the endosomes, or will they continue to signal in the endosomal compartment. In other cellular systems, endosomes are being analyzed as specific intracellular signaling compartments that permit differential spread of downstream pathways from receptors [44]. This is an attractive hypothesis that might also be valid for the IS.
Late endosomes: the multivesicular bodies
Multivesicular bodies (MVBs) are an intermediate compartment where macromolecules are sorted for recycling or directed towards degradation. The production of MVBs is a multistep process that includes changes in lipid composition and acidification of the endosomal compartment. This is in part accomplished through the traffic of some MVB-specific components from the trans-Golgi network (TGN) to their final destination, such as lysosomes [44]. MVBs can also move to the nascent IS (Figure 1) [10, 55, 56], where they have been implicated in massive actin polymerization, exocytosis of exosomes, and in the formation of lytic granules. We provide evidence to support the role of MVBs at the IS below.
By recruiting the clathrin adaptor Hrs, localization of MVBs to the IS may help organize a dense actin network at the IS. This recruitment may promote massive polymerization of actin filaments from the MVB [56]. This actin network promotes sustained T cell activation by allowing the rapid movement of vesicles and organelles underneath the IS and by possibly directing the movement of receptors located at the plasma membrane [57].
In addition to their role in actin polymerization, MVBs are also associated with exocytosis. MVBs are rich in lysobiphosphatidic acid (LBPA), which is also found in the cSMAC plasma membrane [58], suggesting a process of docking and fusion of MVBs at the IS. Upon fusion at the IS, the contents of MVBs undergo exocytosis. MVBs in the T cell support the active secretion of microRNA-loaded exosomes towards contacting APCs [10]. The microRNA cargo is functional in the APC and some miRNAs have been shown to control the immune response [59, 60], representing a novel mechanism for cell-cell communication and immune modulation. Moreover, the lipid composition of the limiting (outer) membrane of the MVB can act as a sensor to enhance or decrease exocytosis. In this sense, DGK, a Ca2+-activated diacylglycerol kinase that acts as a negative regulator of T cell activation through the production of phosphatidic acid [61], may negatively regulate TCR-triggered signaling by impairing MVBs role as a recycling/sorting compartment. Studies showed that lack of DGK resulted in increased exosome release by stimulated T cells [55] and prevented T cell anergy upon CD3 stimulation without costimulation [61].
MVBs are also strongly implicated in the formation of lytic granules in CTLs differentiated from CD8+T cells. Lytic granules are enriched in Rab27a, which assists in their translocation from the centrosome to the plasma membrane where they release granzyme, perforins and other effector molecules that kills the target cell [21]. The SNARE Synaptobrevin 2 facilitates the fusion of lytic granules with the plasma membrane of human CTLs [62]. The strength of the TCR signal controls the focal point for granule exocytosis, permitting release at the pSMAC if the signal is weak instead of delivery to the secretory domain at the cSMAC [63]. Upon centrosomal relocalization, lytics granules are delivered to the IS by a complex formed by kinesin-1/Rab27a/Slp3 [64], suggesting lytic granules depend on microtubules. This movement is essential to allow the formation of the so-called secretory domain, which favours the focused exocytosis of lytic granule content at the contact area with the target cell. In addition, Myosin IIA can bind to lytic granules, which is dependent on the correct phosphorylation at the S1943 residue [65]. Myosin allows their movement and distribution throughout the actin cytoskeleton. Therefore, centrosomal reorientation and F-actin and microtubular network organization at the IS provides strong evidence for the role of MVBs and related vesicles as major orchestrators at the IS.
The mitochondrial network: how to fuel the IS
Mitochondria actively relocalize to sites with a high demand for ATP or where buffering of intracellular Ca2+ flux is needed. Their transport along the tubulin cytoskeleton by kinesin and dynein motor proteins is well known in different systems, as is the fusion and fission dynamics of the mitochondrial network [66]. In T lymphocytes, mitochondria preferentially localize to the vicinity of the IS upon TCR activation. Here they regulate calcium signaling and orchestrate IS formation and stability (Figure 1 and 3), two important factors needed for T cell activation. The movement and localization of mitochondria towards the IS depends on integrin adhesion and cell polarization [5, 67, 68]. The mitochondrial fission factor Drp1 helps localize mitochondria towards the nascent IS to allow mitochondria-dependent regulation of energy supply and IS architecture (Figure 3 and Box 2). Impaired Drp1 function decreases the actomyosin-dependent centripetal flux of the TCR to the cSMAC at the plasma membrane, thereby increasing persistent TCR activity within microclusters, proximal TCR signalling, and IL-2 production. Therefore, mitochondria not only regulate the strength of T cell activation but also terminate IS signaling [5].
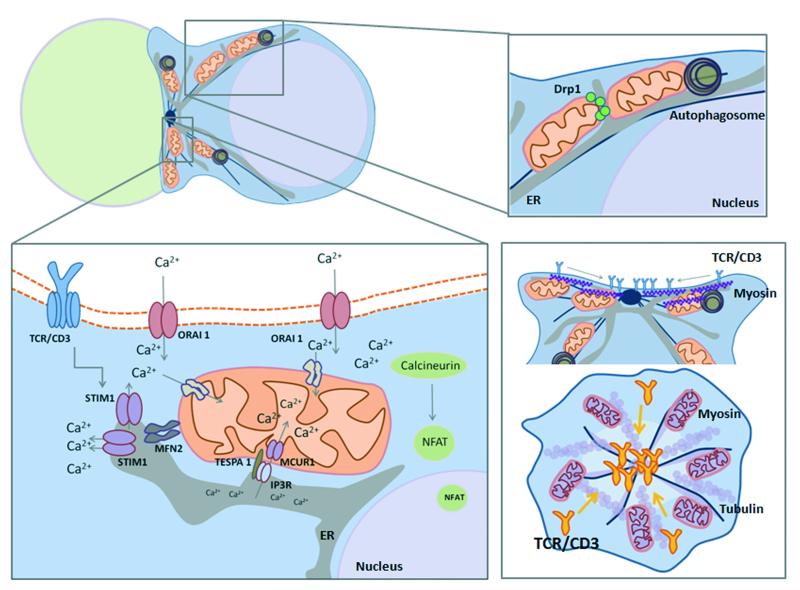
Figure 3. Mitochondrial polarization: fueling the immune synapse.
Mitochondrial fission factor Drp1 localizes mitochondria towards the nascent IS. Increased ATP levels at the IS are needed for energy-consuming signalling and for the acto-myosin centripetal flux of TCR to the central area. The tethering of mitochondria with the endoplasmic reticulum (ER) is relevant for sustained Ca2+ influx upon TCR activation. Mitochondria avoid premature Ca2+-dependent inactivation of CRAC/ORAI1 channels and calcium flux across plasma membrane. Mitofusin-2-dependent mitochondria–ER tethering modulates trafficking of STIM1 to activate ORAI1. Additionally, Junctate has been proposed as an alternate Ca2+-sensing ER protein that regulates recruitment of stromal interaction molecule 1 (STIM1) [94]. Mitochondrial-Associated ER Membranes (MAMs) define the position of mitochondrial division sites where ER tubules contact mitochondria and mediate constriction before Drp1 recruitment [95]. MAMs regulate autophagosome formation through mitochondrial calcium uptake, which regulates cell bioenergetics and inhibits constitutive autophagy [72]. Tespa1 links IP3R-mediated Ca2+ release and mitochondrial Ca2+ uptake in the MAM compartment [96]. MCUR1 functions as a uniporter channel essential for calcium uptake in mitochondria [97]. TCR stimulation promotes autophagy and formation of autophagosomes. Autophagy regulates T cell homeostasis by promoting T lymphocyte survival and proliferation by orchestrating mitochondrial clearance in T cells. ER compartment is increased in autophagy-deficient T lymphocytes, which display a defective influx of calcium from expanded ER stores [98]. Impairing Drp1 function decreases the centripetal flux of the TCR to the central area through impairment of actomyosin contraction and maintains TCR activity, and promotes proximal TCR signalling and IL-2 production. Mitochondria play a dual role at the IS by regulating the strength of T cell activation and the termination of the IS signalling.
Box 2. Mitochondria, ROS and nuclear activation.
The polarized localization of mitochondria at the IS might create a gradient of reactive oxygen species (ROS) in the cell. Regulation of ROS levels by the mitochondrial antioxidant enzyme, manganese superoxide dismutase (MnSOD/SOD2) modulates NF-κB- and AP-1-mediated transcription [91]. During T cell activation, mitochondrial ROS are generated at mitochondrial complexes I and III. Downregulation of the complex I chaperone NDUFAF1 impairs TCR-triggered ROS generation and IL-2 and IL-4 secretion. The Rieske iron-sulfur protein (RISP) of complex III is encoded by uqcrfs1. uqcrfs1−/− CD4+ T cells fail to induce IL-2, CD69 and CD25 due to decreased NFAT1 translocation to the nucleus [92]. Oxidative signals can be regulated by the TCR-triggered activation of ADP-dependent glucokinase (ADPGK), which increases glycolytic metabolism and diverts glycolysis toward the mitochondrial glycerol-3-phosphate dehydrogenase (GPD) shuttle. The activation of GPD2 results in hyperreduction of ubiquinone and release of ROS from mitochondria, resulting in the upregulation of NF-κB-dependent gene expression [93]. Mitochondrial ROS signaling is thus required for antigen-specific T cell activation, highlighting the critical importance of mitochondrial metabolism in the regulation of the T cell nuclear program.
The tethering of mitochondria to the endoplasmic reticulum (ER) is also important for sustained Ca2+ influx upon TCR activation [9, 69]. This function requires interaction of STIM1, a Ca2+ sensor of store depletion in the ER, with Orai1, a pore subunit of plasma membrane CRAC channels. Upon ER Ca2+ store depletion, STIM1 translocates and co-clusters with Orai1 in proximity to the plasma membrane and the ER. This interaction facilitates the opening of CRAC channels and results in calcium flux across the plasma membrane. To avoid premature Ca2+-dependent inactivation of CRAC/Orai1 channels, mitochondria act as inflowing Ca2+ buffers [70] and localize to the vicinity of the IS [68], a crucial event in the regulation of T cell activation responses (Figure 3, and Box 1). Orai1-induced calcium influx is required for degranulation of CTLs [71]. The dynamics of ER and mitochondria are tightly coupled and these organelles have extensive contacts at junctions called mitochondria-associated ER membranes (MAMs). MAMs regulate central aspects of cell homeostasis including cell bioenergetics, formation of autophagosomes, positioning of mitochondrial division, and regulation of apoptosis [72]. MAMs have been identified at the IS, and mitochondrial proteins have been found at the cSMAC [73]. Therefore, the presence of MAMs and organelles at the IS allows the interchange of information between organelles and the plasma membrane, which may represent a type of ‘cell memory’ of activation, therefore facilitating a second challenge in the case of short contacts between the T cell and the APC, or the prevention of further activation events.
Implications for the propagation of signals to the nucleus
Upon TCR engagement with an APC, activation signals propagate to the nucleus and increase the transcription of T cell differentiation genes. The activation of nuclear genes involved in T cell differentiation is an active area of research. At the IS of the conjugated T cell, the nucleus is located behind the centrosome and connected via microtubules. Materials are shuttled between the cytoplasm and the nucleus through highly regulated nuclear pores (Figures 1 and 3). Ran-GTPases located at the centrosome of interphase cells participate in this import and export of proteins from the nucleus [11]. The compartmentalization of signals in organelles may therefore regulate how and when a pathway can reach the nucleus and activate or deactivate target genes. In this regard, binding of the adaptor protein Bcl10 to the autophagy adaptor p62 is essential for Bcl10-mediated NF-kB activation in effector and naïve T cells; however, fine-tuned activation of NF-kB is achieved only in effector T cells, through selective, autophagy-mediated degradation of ubiquitinated p62-bound Bcl10 [74]. Selective activation or potentiation of similar pathways during T cell differentiation provides the cell with new tools to regulate nuclear activation. Indeed, anergy, possibly the paradigm of signaling divergence, is regulated by several mechanisms. GRAIL (gene related to anergy in lymphocytes), a known gatekeeper for T cell anergy, is associated with the transferrin-endocytosis recycling system [75], and an example of how compartmentalization can regulate T cell activation. Anergy, tolerance regulation, and T cell hyper-responsiveness have been shown to be regulated by Cbl-b and Itch. Cbl-b is a RING-E3 ligase for several intracellular molecules and cooperates with the HECT-E3 ligase Itch to ubiquitinate CD3ζ without promoting its degradation [76]. Furthermore, Itch has been shown to ubiquitinate the Jun family proteins c-Jun and JunB, thereby down-regulating T helper type 2 (Th2) cell cytokine production and proliferation [47]. Cbl is recruited to LAT/SLP76 signalosomes, therefore concentrating its ubiquitination action at the IS. Therefore, subsynaptical vesicles implicated in signaling may regulate T cell activation by transporting active Cbl to the cell interior, consequently facilitating protein ubiquitination and sorting to degradative or recycling pathways.
Asymmetric cell division: the ultimate polarization
Sustained synapses are critical for T cell activation at the initiation of antigen presentation and provide a cue for the establishment and maintenance of polarity throughout cell division. Persistent polarization can result in asymmetric segregation of fate-determining proteins during cell division, providing T cells with the ability to generate lineage diversity during the course of the immune response (Figure 4). The clonal selection theory of adaptive immunity suggests that during immune responses, cell diversity is generated early from a single naïve cell that gives rise to both effector and memory cells [77]. In order for T cells to undergo asymmetric cell division, prolonged interactions with APCs in vivo are required [78]. However, whether T cells can divide asymmetrically while in contact with an APC has only been demonstrated in vitro [13].
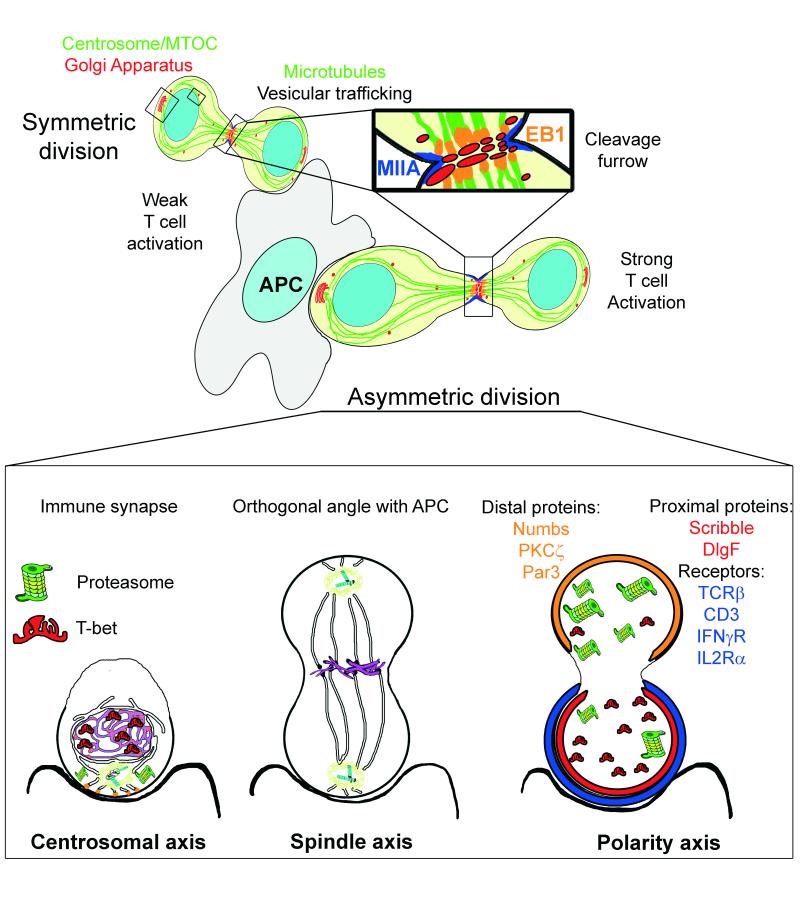
Figure 4. Asymmetric cell division in T cells.
T cells can divide asymmetrically while in contact with an APC in vitro. Sustained synapses promoted by strong antigenic stimulation are critical for T cell activation and provide a cue for the establishment and maintenance of polarity throughout cell division. The cell asymmetry of proteins and organelles obtained through the challenge of the IS may be useful to conform an axis of polarity during cell division. The initial localization of the centrosome may be a marker to organize the spindle axis once the centrioles have duplicated. The polarity axis conformed by the IS can be reinforced by vesicular traffic from the Golgi towards the cleavage furrow during cell division. This traffic can promote the accumulation of proteins in one of the cells. EB1 parallels its own localization at the IS in the cleavage furrow. T cell receptors are unequally divided among daughter cells. CD4 naïve T cells promote TCRβ, IFNγR and IL2Rα polarization toward the IS and subsequently, the proximal cell [99]. CD3 and IL2Rα are similarly recruited in CD8 memory cells [100]. The polarity proteins known as partitioning defective (Par) proteins consist of the Par3-Par6-atypical PKC (aPKC) and Discs large (Dlg)-Scribble-Lethal giant larvae (Lgl) polarity complexes and are differentially distributed during asymmetric cell division, as is Numb, an endocytic adaptor that can also help to regulate polarity [80]. Indeed, several transcriptional regulators undergo asymmetric segregation in lymphocytes. T-bet, which drives effector differentiation in CD8+ T cells and the Th1 fate in CD4+ T cells is unequally inherited during division of both types of lymphocytes [101]. The asymmetric segregation of the proteasome determines the preferential degradation of T-bet, yielding one daughter cell that inherits more T-bet. These results suggest a mechanism during IS formation where a cell may unequally localize cellular activities during division, thereby imparting disparity in the abundance of cell fate regulators in the daughter cells.
Asymmetric cell division requires the alignment of the mitotic spindle with the axis of polarity. In activated T cells, the organization of this axis starts with TCR activation and polarization at the IS, which leads to centrosome and organelle redistribution towards the APC (Figure 4). This axis of polarity during IS formation is regulated by members of an evolutionary conserved network of polarity proteins, called Par proteins, which include the Par3-Par6-atypical PKC (aPKC) and Dlg-Scrib-Lgl polarity complexes. Par1/EMK/MARK2 is a Ser/Thr kinase localized at the plasma membrane. Wild-type Par1 polarizes to the IS upon TCR activation, where it is phosphorylated by PKC-ζ and found in the cytosol. In contrast, a kinase-dead mutant form of Par1 remains randomly distributed at the T cell plasma membrane [79] suggesting Par1 regulates centrosome polarization. Furthermore, Dlg appears to regulate the microtubule network at the nascent IS [19]. Therefore, these proteins may control the cytoskeleton organization to establish the polarity axis prior to the duplication of the centrioles and the progression of the cell cycle. After duplication, one of the centrosomes remains anchored by microtubules at the initial position, whereas the second relocates to the opposite side of the nucleus, before the breakdown of the nuclear envelope [11]. At a persistent IS, centrosome maturation and duplication take place at this position promoting the orthogonal alignment of the spindle axis to the APC contact. This alignment is regulated by the polarity complexes Dlg and Partner of Inscuteable (Pins) [12]. Scribble and aPKC are asymmetrically distributed in mitotic T cells responding to Listeria infection [80]. aPKC and Par3 polarize to the distal side of the cell in early mitotic T cells and maintain this asymmetry during late mitosis. In contrast, Scribble and Dlg are both polarized to the proximal cell during early and late mitosis. However, Scribble defective B lymphocytes do not show any evident defects in asymmetric division and differentiation, which points to different requirements for asymmetric cell division in populations of CD45+ cells, such as T and B cells [81]. The organization of the cytoskeleton during late mitosis serves to facilitate vesicular trafficking between the two daughter cells (Figure 4). A variety of determinants can be similarly asymmetrically inherited including proteins, organelles and membrane components [11]. Therefore, the correct positioning of different organelles early upon TCR activation may determine the localization and orientation of the polarity axis and mitotic spindle, constituting a positive feedback for TCR signalling and favouring T cell asymmetric division.
Concluding remarks
The immune system requires the existence of different T cell subtypes and populations to modulate responses or act as effector cells. The process of T cell activation involves more than the antigenic stimulation of receptors at the cell surface; the intracellular partitioning of the signal is also required. New findings support the existence of activated CD3 and LAT complexes integrated in vesicles that may constitute a new pathway for activation. This activation, when appropriate, leads to the proliferation of T cells, which can be symmetric or asymmetric. Polarization of organelles toward the immune synapse is more intense when the signal is stronger, and may therefore correlate with asymmetry in cell division. The positioning of the centrosome at the immune synapse may determine the polarity axis and the spindle axis, promoting asymmetric cell division. Polarization of the mitochondrial network may contribute to this effect by generating focal concentrations of intracellular calcium, ROS and ATP [82]. Therefore, immune synapse polarity may initiate the polarization of the mother and daughter cell during T cell division. CD8+ memory T cells have more mitochondrial biogenesis, fatty-acid oxidation and mitochondrial spare respiratory capacity (SRC) than naïve or effector T cells. Nevertheless, how this qualitative mitochondrial diversity is created is uncertain. An attractive hypothesis is that mitochondria are unequally partitioned during asymmetric cell division.
Acknowledgements
The authors would like to apologize to those colleagues whose work could not be cited here owing to space limitations. We thank S. Bartlett for editorial support and critical reading of the manuscript. This work was supported by grants to FSM from Spanish Ministry of Science and Innovation (SAF2011-25834), Instituto Salud Carlos III (Red Cardiovascular RD12-0042-0056), Comunidad de Madrid (INDISNET S2011/BMD-2332) and European Union (ERC-2011-AdG 294340-GENTRIS; grant to FSM and FB and NBMC support). The Centro Nacional de Investigaciones Cardiovasculares (CNIC, Spain) is supported by the Spanish Ministry of Science and Innovation and the Pro-CNIC Foundation.
Glossary
- Antigen presenting cell (APC)
Express major histocompatibility complex (MHC) on its surface. So-called professional APCs express MHC type II, recognized by the CD4 molecule, whereas all cell types express MHC type I, recognized by the CD8 molecule. MHC is processed to form a complex with the peptide sequence known as antigen and sorted to the plasma membrane to be recognized by the TCR, therefore setting the basis for adaptive immunity.
- Asymmetric cell division
cell division that results in a daughter cell segregated from the mother cell that shows divergent cell-fate.
- Centrosome
a microtubule-organizing center (MTOC) formed by a pair of centrioles surrounded by an amorphous material termed pericentrosomal material. It is in charge of microtubule polymerization and Golgi cisternae organization in interphase cells. MTOC helps to organize the microtubule cytoskeleton through the nucleation of the heterodimers of α and β tubulin and prevents their massive depolymerization by capping the highly unstable, minus free-ends of microtubules.
- Cytotoxic lymphocytes (CTL)
CD8+ T cells that promote the killing of a target cell bearing a pMHC-I complex recognized by the TCR. It promotes killing via the polarized secretion of lytic granules containing perforin and proteolytic enzymes, primarily granzymes and cathepsins.
- Endosomes
Vesicles formed by endocytosis are rapidly targeted to the early endosomal compartment, a tubulovesicular network that spread throughout the cortical cytoplasm. The incorporation of different components from the Golgi and acidification of the lumen matures early into late endosomes.
- Immune synapse
a highly-organized molecular structure formed at the cell-cell contact area between a T cell and an antigen-presenting cell upon antigen recognition by the T cell. It relies on receptor clustering at the plasma membrane and cytoskeleton segregation to stabilize the interaction of both cells. The immune synapse serves to promote the correct activation, proliferation and differentiation of T cells, allowing them to carry out their effector functions.
- Immune-receptor Tyrosine-based Activation Motif (ITAM)
This is a conserved aminoacidic sequence present in all CD3 subunits at their cytoplasmic tails. CD3ζ contains three of them. TCR stimulation phosphorylates ITAMs where they act as docking sites for SH2-domain containing proteins, facilitating the formation of signalosomes.
- Linker for activation of T cells (LAT)
This is a scaffold molecule containing several tyrosine residues phosphorylated upon TCR activation. These residues serve as docking sites for different signalling proteins to form LAT signalosomes.
- Lymphocyte-function associated antigen 1 (LFA-1)
An αLβ2 integrin present in lymphoid cells that promotes cell-cell adhesion through specific binding to its ligand, ICAM-1 (Intercellular adhesion molecule-1).
- Memory T cells
subpopulation of long-term T cells that have previously encountered and responded to their cognate antigen. At a second challenge with the antigen, memory T cells proliferate to mount a faster and stronger immune response. Asymmetric division allows the maintenance of memory and effector CD4+ or CD8+ T cells.
- Microclusters
protein islands formed at the plasma membrane or below it by a small number of units of the same molecule that can coalesce into complex congregates and typically contains a specific signalosome.
- Microtubule
rigid hollow cylinder of approximately 25 nm of diameter, formed typically by 13 protofilaments of tubulin heterodimers, organized in a helical disposition. Because of the dimeric character of tubulin and the strong alternation of α and β isoforms, microtubules show polar distribution, where the equilibrium between incorporation and loss of α/β-tubulin is greater at the plus end than at the minus end.
- Multivesicular bodies (MVBs)
Organelles consisting of late endosomes that contain luminal vesicles, formed upon the inward budding of the outer endosomal membrane. The limiting (outer) membrane of MVBs can either fuse with lysosomes to promote degradation or fuse to the plasma membrane to release their content to the extracellular medium.
- Naïve T cells
subset of mature, circulating T cells that can activate and proliferate upon their encounter with antigens recognized by their specific TCR. To mount an immune response, they produce effector and memory T cells through asymmetric cell division.
- Signalosomes
signaling modules composed by interconnected proteins that serve to concentrate the initial activation by a specific cue and to propagate and magnify it to a specific cell location. Proteins containing SH2 and SH3 (Src homology 2 and 3) domains or their corresponding docking sites—phosphorylated tyrosine residues and poly-proline sequences, respectively—typically form part of them.
- Src homology 2 (SH2) domain-containing leukocyte protein of 76 (SLP76)
Ths is a 76kDa scaffold protein that can form microclusters below the plasma membrane. SLP76 interacts with different proteins through several tyrosine residues that are phosphorylated upon TCR activation, a proline-rich domain and a SH2 domain and therefore organizes its own signalosome.
- Symmetric cell division
mitosis or cell division that involves an equal distribution of cellular components and results in two daughter cells with characteristics similar to the mother cell.
- T cell
T cells are lymphoid cells characterized by the presence of the T cell receptor (TCR) and its associated CD3 complex. Mature T cells can express either the CD8 or CD4 molecules as co-receptor for MHC-I or MHC-II-peptide complex.
- T cell Receptor (TCR)
This receptor is formed by heterodimers of ligand-binding subunits, the α and β (αβ T cells) or γ and δ subunits (γδ T cells), which associate to the signal-transducing subunits CD3γ, δ, ε and ζ. The TCR is in charge of specific antigenic recognition in the context of MHC (pMHC). TCR activation by the pMHC promotes immune synapse formation.
- Tubulin
A family of globular proteins that are the main constituents of microtubular structures. The most common members of the family are the α, β and γ isoforms, each of which correspond to different genes. In its native state, α and β isoforms exist as heterodimers that form the microtubules. Several γ-tubulin molecules can be found in protein complexes known as γ-tubulin ring complexes (γ-TuRCs), which chemically mimic the plus end of microtubules, allowing polymerization. γ-TuRCs are primarily found at cellular MTOCs.
Footnotes
Conflict of Interest Statement. The Authors declare that there is no conflict of interest.
References
- 1.Lasserre R, Alcover A. Cytoskeletal cross-talk in the control of T cell antigen receptor signaling. FEBS Lett. 2010;584:4845–4850. doi: 10.1016/j.febslet.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Martin-Cofreces NB, et al. Tubulin and actin interplay at the T cell and antigen-presenting cell interface. Front Immunol. 2011;2:24. doi: 10.3389/fimmu.2011.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaw AS, Filbert EL. Scaffold proteins and immune-cell signalling. Nat Rev Immunol. 2009;9:47–56. doi: 10.1038/nri2473. [DOI] [PubMed] [Google Scholar]
- 4.Yanez-Mo M, et al. Tetraspanin-enriched microdomains: a functional unit in cell plasma membranes. Trends Cell Biol. 2009;19:434–446. doi: 10.1016/j.tcb.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Baixauli F, et al. The mitochondrial fission factor dynamin-related protein 1 modulates T-cell receptor signalling at the immune synapse. EMBO J. 2011;30:1238–1250. doi: 10.1038/emboj.2011.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huse M, et al. Shouts, whispers and the kiss of death: directional secretion in T cells. Nature immunology. 2008;9:1105–1111. doi: 10.1038/ni.f.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kupfer A, Singer SJ. The specific interaction of helper T cells and antigen-presenting B cells. IV. Membrane and cytoskeletal reorganizations in the bound T cell as a function of antigen dose. The Journal of experimental medicine. 1989;170:1697–1713. doi: 10.1084/jem.170.5.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kupfer A, Dennert G. Reorientation of the microtubule-organizing center and the Golgi apparatus in cloned cytotoxic lymphocytes triggered by binding to lysable target cells. Journal of immunology. 1984;133:2762–2766. [PubMed] [Google Scholar]
- 9.Quintana A, et al. Calcium microdomains at the immunological synapse: how ORAI channels, mitochondria and calcium pumps generate local calcium signals for efficient T-cell activation. EMBO J. 2011;30:3895–3912. doi: 10.1038/emboj.2011.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mittelbrunn M, et al. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat Commun. 2011;2:282. doi: 10.1038/ncomms1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knoblich JA. Mechanisms of asymmetric stem cell division. Cell. 2008;132:583–597. doi: 10.1016/j.cell.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 12.Russell S. How polarity shapes the destiny of T cells. J Cell Sci. 2008;121:131–136. doi: 10.1242/jcs.021253. [DOI] [PubMed] [Google Scholar]
- 13.King CG, et al. T cell affinity regulates asymmetric division, effector cell differentiation, and tissue pathology. Immunity. 2012;37:709–720. doi: 10.1016/j.immuni.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Witte H, et al. Microtubule stabilization specifies initial neuronal polarization. J Cell Biol. 2008;180:619–632. doi: 10.1083/jcb.200707042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li R, Gundersen GG. Beyond polymer polarity: how the cytoskeleton builds a polarized cell. Nat Rev Mol Cell Biol. 2008;9:860–873. doi: 10.1038/nrm2522. [DOI] [PubMed] [Google Scholar]
- 16.Conde C, Caceres A. Microtubule assembly, organization and dynamics in axons and dendrites. Nat Rev Neurosci. 2009;10:319–332. doi: 10.1038/nrn2631. [DOI] [PubMed] [Google Scholar]
- 17.Martin-Cofreces NB, et al. MTOC translocation modulates IS formation and controls sustained T cell signaling. J Cell Biol. 2008;182:951–962. doi: 10.1083/jcb.200801014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin-Cofreces NB, et al. End-binding protein 1 controls signal propagation from the T cell receptor. EMBO J. 2012;31:4140–4152. doi: 10.1038/emboj.2012.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lasserre R, et al. Ezrin tunes T-cell activation by controlling Dlg1 and microtubule positioning at the immunological synapse. EMBO J. 2010;29:2301–2314. doi: 10.1038/emboj.2010.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lasserre R, et al. Release of serine/threonine-phosphorylated adaptors from signaling microclusters down-regulates T cell activation. J Cell Biol. 2011;195:839–853. doi: 10.1083/jcb.201103105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Angus KL, Griffiths GM. Cell polarisation and the immunological synapse. Curr Opin Cell Biol. 2012;25:85–91. doi: 10.1016/j.ceb.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chemin K, et al. Cytokine secretion by CD4+ T cells at the immunological synapse requires Cdc42-dependent local actin remodeling but not microtubule organizing center polarity. J Immunol. 2012;189:2159–2168. doi: 10.4049/jimmunol.1200156. [DOI] [PubMed] [Google Scholar]
- 23.Bertrand F, et al. An initial and rapid step of lytic granule secretion precedes microtubule organizing center polarization at the cytotoxic T lymphocyte/target cell synapse. Proc Natl Acad Sci U S A. 2013;110:6073–6078. doi: 10.1073/pnas.1218640110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jenkins MR, et al. The strength of T cell receptor signal controls the polarization of cytotoxic machinery to the immunological synapse. Immunity. 2009;31:621–631. doi: 10.1016/j.immuni.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin-Cofreces NB, et al. Role of Fyn in the rearrangement of tubulin cytoskeleton induced through TCR. J Immunol. 2006;176:4201–4207. doi: 10.4049/jimmunol.176.7.4201. [DOI] [PubMed] [Google Scholar]
- 26.Tsun A, et al. Centrosome docking at the immunological synapse is controlled by Lck signaling. J Cell Biol. 2011;192:663–674. doi: 10.1083/jcb.201008140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quann EJ, et al. Localized diacylglycerol drives the polarization of the microtubule-organizing center in T cells. Nat Immunol. 2009;10:627–635. doi: 10.1038/ni.1734. [DOI] [PubMed] [Google Scholar]
- 28.Vicente-Manzanares M, Sanchez-Madrid F. Role of the cytoskeleton during leukocyte responses. Nat Rev Immunol. 2004;4:110–122. doi: 10.1038/nri1268. [DOI] [PubMed] [Google Scholar]
- 29.Robles-Valero J, et al. Integrin and CD3/TCR activation are regulated by the scaffold protein AKAP450. Blood. 2010;115:4174–4184. doi: 10.1182/blood-2009-12-256222. [DOI] [PubMed] [Google Scholar]
- 30.Balagopalan L, et al. The LAT story: a tale of cooperativity, coordination, and choreography. Cold Spring Harb Perspect Biol. 2010;2:a005512. doi: 10.1101/cshperspect.a005512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bonello G, et al. Dynamic recruitment of the adaptor protein LAT: LAT exists in two distinct intracellular pools and controls its own recruitment. J Cell Sci. 2004;117:1009–1016. doi: 10.1242/jcs.00968. [DOI] [PubMed] [Google Scholar]
- 32.Lillemeier BF, et al. TCR and Lat are expressed on separate protein islands on T cell membranes and concatenate during activation. Nat Immunol. 2009;11:90–96. doi: 10.1038/ni.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Purbhoo MA, et al. Dynamics of subsynaptic vesicles and surface microclusters at the immunological synapse. Sci Signal. 2010;3:ra36. doi: 10.1126/scisignal.2000645. [DOI] [PubMed] [Google Scholar]
- 34.Williamson DJ, et al. Pre-existing clusters of the adaptor Lat do not participate in early T cell signaling events. Nat Immunol. 2011;12:655–662. doi: 10.1038/ni.2049. [DOI] [PubMed] [Google Scholar]
- 35.Sherman E, et al. Functional nanoscale organization of signaling molecules downstream of the T cell antigen receptor. Immunity. 2011;35:705–720. doi: 10.1016/j.immuni.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu H, et al. SH2 domain containing leukocyte phosphoprotein of 76-kDa (SLP-76) feedback regulation of ZAP-70 microclustering. Proc Natl Acad Sci U S A. 2010;107:10166–10171. doi: 10.1073/pnas.0909112107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barda-Saad M, et al. Cooperative interactions at the SLP-76 complex are critical for actin polymerization. EMBO J. 2010;29:2315–2328. doi: 10.1038/emboj.2010.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Das V, et al. Activation-induced polarized recycling targets T cell antigen receptors to the immunological synapse; involvement of SNARE complexes. Immunity. 2004;20:577–588. doi: 10.1016/s1074-7613(04)00106-2. [DOI] [PubMed] [Google Scholar]
- 39.Alcover A, Thoulouze MI. Vesicle traffic to the immunological synapse: a multifunctional process targeted by lymphotropic viruses. Curr Top Microbiol Immunol. 2009;340:191–207. doi: 10.1007/978-3-642-03858-7_10. [DOI] [PubMed] [Google Scholar]
- 40.Dustin ML, Depoil D. New insights into the T cell synapse from single molecule techniques. Nat Rev Immunol. 2011;11:672–684. doi: 10.1038/nri3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Larghi P, et al. VAMP7 controls T cell activation by regulating the recruitment and phosphorylation of vesicular Lat at TCR-activation sites. Nat Immunol. 2013 doi: 10.1038/ni.2609. [DOI] [PubMed] [Google Scholar]
- 42.Oddos S, et al. High-speed high-resolution imaging of intercellular immune synapses using optical tweezers. Biophys J. 2008;95:L66–68. doi: 10.1529/biophysj.108.143198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yudushkin IA, Vale RD. Imaging T-cell receptor activation reveals accumulation of tyrosine-phosphorylated CD3zeta in the endosomal compartment. Proc Natl Acad Sci U S A. 2010;107:22128–22133. doi: 10.1073/pnas.1016388108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murphy JE, et al. Endosomes: a legitimate platform for the signaling train. Proc Natl Acad Sci U S A. 2009;106:17615–17622. doi: 10.1073/pnas.0906541106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gorska MM, et al. Uncoordinated 119 protein controls trafficking of Lck via the Rab11 endosome and is critical for immunological synapse formation. J Immunol. 2009;183:1675–1684. doi: 10.4049/jimmunol.0900792. [DOI] [PubMed] [Google Scholar]
- 46.Andres-Delgado L, et al. INF2 promotes the formation of detyrosinated microtubules necessary for centrosome reorientation in T cells. J Cell Biol. 2012;198:1025–1037. doi: 10.1083/jcb.201202137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bhoj VG, Chen ZJ. Ubiquitylation in innate and adaptive immunity. Nature. 2009;458:430–437. doi: 10.1038/nature07959. [DOI] [PubMed] [Google Scholar]
- 48.Vardhana S, et al. Essential role of ubiquitin and TSG101 protein in formation and function of the central supramolecular activation cluster. Immunity. 2010;32:531–540. doi: 10.1016/j.immuni.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Patino-Lopez G, et al. Rab35 and its GAP EPI64C in T cells regulate receptor recycling and immunological synapse formation. J Biol Chem. 2008;283:18323–18330. doi: 10.1074/jbc.M800056200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martinez-Martin N, et al. T cell receptor internalization from the immunological synapse is mediated by TC21 and RhoG GTPase-dependent phagocytosis. Immunity. 2011;35:208–222. doi: 10.1016/j.immuni.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qureshi OS, et al. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011;332:600–603. doi: 10.1126/science.1202947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yokosuka T, et al. Spatiotemporal basis of CTLA-4 costimulatory molecule-mediated negative regulation of T cell activation. Immunity. 2010;33:326–339. doi: 10.1016/j.immuni.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 53.Nurieva RI, et al. The E3 ubiquitin ligase GRAIL regulates T cell tolerance and regulatory T cell function by mediating T cell receptor-CD3 degradation. Immunity. 2010;32:670–680. doi: 10.1016/j.immuni.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Balagopalan L, et al. Enhanced T-cell signaling in cells bearing linker for activation of T-cell (LAT) molecules resistant to ubiquitylation. Proc Natl Acad Sci U S A. 2011;108:2885–2890. doi: 10.1073/pnas.1007098108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alonso R, et al. Diacylglycerol kinase alpha regulates the formation and polarisation of mature multivesicular bodies involved in the secretion of Fas ligand-containing exosomes in T lymphocytes. Cell Death Differ. 2011;18:1161–1173. doi: 10.1038/cdd.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Calabia-Linares C, et al. Endosomal clathrin drives actin accumulation at the immunological synapse. J Cell Sci. 2011;124:820–830. doi: 10.1242/jcs.078832. [DOI] [PubMed] [Google Scholar]
- 57.Purbhoo MA. The function of sub-synaptic vesicles during T-cell activation. Immunol Rev. 2013;251:36–48. doi: 10.1111/imr.12012. [DOI] [PubMed] [Google Scholar]
- 58.Varma R, et al. T cell receptor-proximal signals are sustained in peripheral microclusters and terminated in the central supramolecular activation cluster. Immunity. 2006;25:117–127. doi: 10.1016/j.immuni.2006.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mittelbrunn M, Sanchez-Madrid F. Intercellular communication: diverse structures for exchange of genetic information. Nat Rev Mol Cell Biol. 2012;13:328–335. doi: 10.1038/nrm3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thery C, et al. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 61.Joshi RP, Koretzky GA. Diacylglycerol kinases: regulated controllers of T cell activation, function, and development. Int J Mol Sci. 2013;14:6649–6673. doi: 10.3390/ijms14046649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Matti U, et al. Synaptobrevin2 is the v-SNARE required for cytotoxic T-lymphocyte lytic granule fusion. Nat Commun. 2013;4:1439. doi: 10.1038/ncomms2467. [DOI] [PubMed] [Google Scholar]
- 63.Beal AM, et al. Kinetics of early T cell receptor signaling regulate the pathway of lytic granule delivery to the secretory domain. Immunity. 2009;31:632–642. doi: 10.1016/j.immuni.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kurowska M, et al. Terminal transport of lytic granules to the immune synapse is mediated by the kinesin-1/Slp3/Rab27a complex. Blood. 2012;119:3879–3889. doi: 10.1182/blood-2011-09-382556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sanborn KB, et al. Myosin IIA associates with NK cell lytic granules to enable their interaction with F-actin and function at the immunological synapse. J Immunol. 2009;182:6969–6984. doi: 10.4049/jimmunol.0804337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hollenbeck PJ, Saxton WM. The axonal transport of mitochondria. J Cell Sci. 2005;118:5411–5419. doi: 10.1242/jcs.02745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Contento RL, et al. Adhesion shapes T cells for prompt and sustained T-cell receptor signalling. EMBO J. 2010;29:4035–4047. doi: 10.1038/emboj.2010.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Quintana A, et al. T cell activation requires mitochondrial translocation to the immunological synapse. Proc Natl Acad Sci U S A. 2007;104:14418–14423. doi: 10.1073/pnas.0703126104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lioudyno MI, et al. Orai1 and STIM1 move to the immunological synapse and are up-regulated during T cell activation. Proc Natl Acad Sci U S A. 2008;105:2011–2016. doi: 10.1073/pnas.0706122105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Parekh AB, Putney JW., Jr. Store-operated calcium channels. Physiol Rev. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- 71.Maul-Pavicic A, et al. ORAI1-mediated calcium influx is required for human cytotoxic lymphocyte degranulation and target cell lysis. Proc Natl Acad Sci U S A. 2011;108:3324–3329. doi: 10.1073/pnas.1013285108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cardenas C, et al. Essential regulation of cell bioenergetics by constitutive InsP3 receptor Ca2+ transfer to mitochondria. Cell. 2010;142:270–283. doi: 10.1016/j.cell.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Christie DA, et al. Mitochondrial and plasma membrane pools of stomatin-like protein 2 coalesce at the immunological synapse during T cell activation. PLoS One. 2012;7:e37144. doi: 10.1371/journal.pone.0037144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Paul S, et al. Selective autophagy of the adaptor protein Bcl10 modulates T cell receptor activation of NF-kappaB. Immunity. 2012;36:947–958. doi: 10.1016/j.immuni.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Whiting CC, et al. GRAIL: a unique mediator of CD4 T-lymphocyte unresponsiveness. FEBS J. 2011;278:47–58. doi: 10.1111/j.1742-4658.2010.07922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huang H, et al. K33-linked polyubiquitination of T cell receptor-zeta regulates proteolysis-independent T cell signaling. Immunity. 2010;33:60–70. doi: 10.1016/j.immuni.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stemberger C, et al. A single naive CD8+ T cell precursor can develop into diverse effector and memory subsets. Immunity. 2007;27:985–997. doi: 10.1016/j.immuni.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 78.Celli S, et al. Real-time manipulation of T cell-dendritic cell interactions in vivo reveals the importance of prolonged contacts for CD4+ T cell activation. Immunity. 2007;27:625–634. doi: 10.1016/j.immuni.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 79.Lin J, et al. The polarity protein Par1b/EMK/MARK2 regulates T cell receptor-induced microtubule-organizing center polarization. J Immunol. 2009;183:1215–1221. doi: 10.4049/jimmunol.0803887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Oliaro J, et al. Asymmetric cell division of T cells upon antigen presentation uses multiple conserved mechanisms. J Immunol. 2010;185:367–375. doi: 10.4049/jimmunol.0903627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hawkins ED, et al. Regulation of asymmetric cell division and polarity by Scribble is not required for humoral immunity. Nat Commun. 2013;4:1801–1811. doi: 10.1038/ncomms2796. [DOI] [PubMed] [Google Scholar]
- 82.van der Windt GJ, et al. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity. 2012;36:68–78. doi: 10.1016/j.immuni.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Akella JS, et al. MEC-17 is an alpha-tubulin acetyltransferase. Nature. 2010;467:218–222. doi: 10.1038/nature09324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nicastro D, et al. Cryo-electron tomography reveals conserved features of doublet microtubules in flagella. Proc Natl Acad Sci U S A. 2011;108:E845–853. doi: 10.1073/pnas.1106178108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kalebic N, et al. Tubulin acetyltransferase alphaTAT1 destabilizes microtubules independently of its acetylation activity. Mol Cell Biol. 2013;33:1114–1123. doi: 10.1128/MCB.01044-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Valenzuela-Fernandez A, et al. HDAC6: a key regulator of cytoskeleton, cell migration and cell-cell interactions. Trends Cell Biol. 2008;18:291–297. doi: 10.1016/j.tcb.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 87.Janke C, Bulinski JC. Post-translational regulation of the microtubule cytoskeleton: mechanisms and functions. Nat Rev Mol Cell Biol. 2011;12:773–786. doi: 10.1038/nrm3227. [DOI] [PubMed] [Google Scholar]
- 88.Peris L, et al. Motor-dependent microtubule disassembly driven by tubulin tyrosination. J Cell Biol. 2009;185:1159–1166. doi: 10.1083/jcb.200902142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schroder JM, et al. EB1 and EB3 promote cilia biogenesis by several centrosome-related mechanisms. J Cell Sci. 2011;124:2539–2551. doi: 10.1242/jcs.085852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Finetti F, et al. Intraflagellar transport is required for polarized recycling of the TCR/CD3 complex to the immune synapse. Nat Cell Biol. 2009;11:1332–1339. doi: 10.1038/ncb1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kaminski MM, et al. Mitochondrial reactive oxygen species control T cell activation by regulating IL-2 and IL-4 expression: mechanism of ciprofloxacin-mediated immunosuppression. J Immunol. 2010;184:4827–4841. doi: 10.4049/jimmunol.0901662. [DOI] [PubMed] [Google Scholar]
- 92.Sena LA, et al. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity. 2013;38:225–236. doi: 10.1016/j.immuni.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kaminski MM, et al. T cell activation is driven by an ADP-dependent glucokinase linking enhanced glycolysis with mitochondrial reactive oxygen species generation. Cell Rep. 2012;2:1300–1315. doi: 10.1016/j.celrep.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 94.Srikanth S, et al. Junctate is a Ca2+-sensing structural component of Orai1 and stromal interaction molecule 1 (STIM1) Proc Natl Acad Sci U S A. 2012;109:8682–8687. doi: 10.1073/pnas.1200667109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Friedman JR, et al. ER tubules mark sites of mitochondrial division. Science. 2011;334:358–362. doi: 10.1126/science.1207385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Matsuzaki H, et al. Tespa1 is a novel component of mitochondria-associated endoplasmic reticulum membranes and affects mitochondrial calcium flux. Biochem Biophys Res Commun. 2013;433:322–326. doi: 10.1016/j.bbrc.2013.02.099. [DOI] [PubMed] [Google Scholar]
- 97.Mallilankaraman K, et al. MCUR1 is an essential component of mitochondrial Ca2+ uptake that regulates cellular metabolism. Nat Cell Biol. 2012;14:1336–1343. doi: 10.1038/ncb2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pua HH, et al. Autophagy is essential for mitochondrial clearance in mature T lymphocytes. J Immunol. 2009;182:4046–4055. doi: 10.4049/jimmunol.0801143. [DOI] [PubMed] [Google Scholar]
- 99.Maldonado RA, et al. Control of T helper cell differentiation through cytokine receptor inclusion in the immunological synapse. J Exp Med. 2009;206:877–892. doi: 10.1084/jem.20082900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ciocca ML, et al. Cutting edge: Asymmetric memory T cell division in response to rechallenge. J Immunol. 2012;188:4145–4148. doi: 10.4049/jimmunol.1200176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chang JT, et al. Asymmetric proteasome segregation as a mechanism for unequal partitioning of the transcription factor T-bet during T lymphocyte division. Immunity. 2011;34:492–504. doi: 10.1016/j.immuni.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]