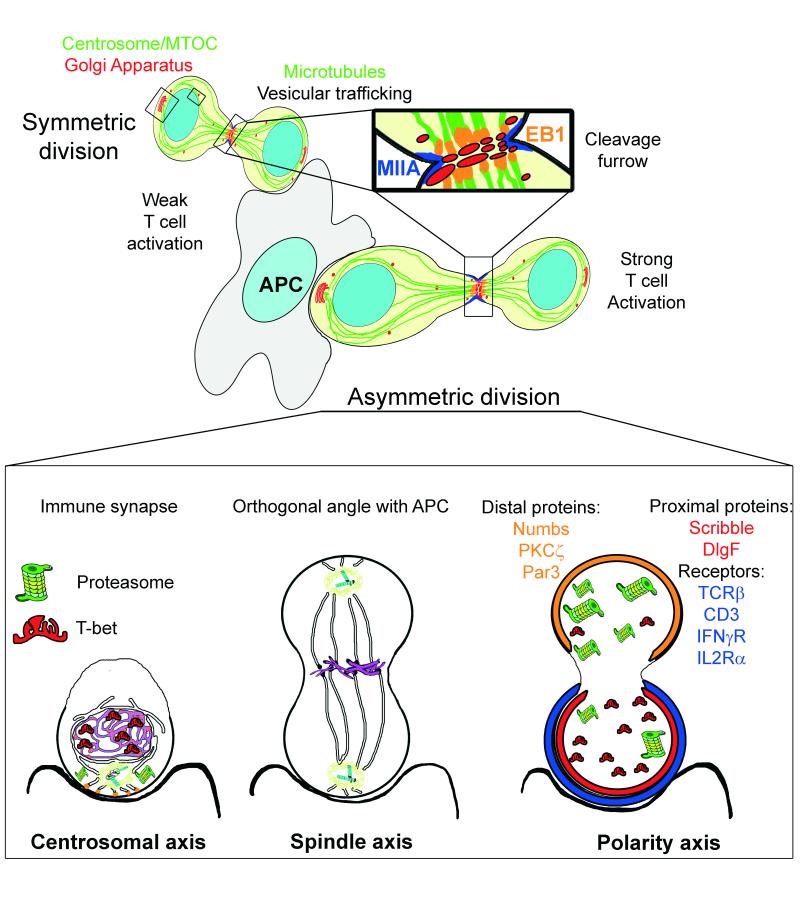
Figure 4. Asymmetric cell division in T cells.
T cells can divide asymmetrically while in contact with an APC in vitro. Sustained synapses promoted by strong antigenic stimulation are critical for T cell activation and provide a cue for the establishment and maintenance of polarity throughout cell division. The cell asymmetry of proteins and organelles obtained through the challenge of the IS may be useful to conform an axis of polarity during cell division. The initial localization of the centrosome may be a marker to organize the spindle axis once the centrioles have duplicated. The polarity axis conformed by the IS can be reinforced by vesicular traffic from the Golgi towards the cleavage furrow during cell division. This traffic can promote the accumulation of proteins in one of the cells. EB1 parallels its own localization at the IS in the cleavage furrow. T cell receptors are unequally divided among daughter cells. CD4 naïve T cells promote TCRβ, IFNγR and IL2Rα polarization toward the IS and subsequently, the proximal cell [99]. CD3 and IL2Rα are similarly recruited in CD8 memory cells [100]. The polarity proteins known as partitioning defective (Par) proteins consist of the Par3-Par6-atypical PKC (aPKC) and Discs large (Dlg)-Scribble-Lethal giant larvae (Lgl) polarity complexes and are differentially distributed during asymmetric cell division, as is Numb, an endocytic adaptor that can also help to regulate polarity [80]. Indeed, several transcriptional regulators undergo asymmetric segregation in lymphocytes. T-bet, which drives effector differentiation in CD8+ T cells and the Th1 fate in CD4+ T cells is unequally inherited during division of both types of lymphocytes [101]. The asymmetric segregation of the proteasome determines the preferential degradation of T-bet, yielding one daughter cell that inherits more T-bet. These results suggest a mechanism during IS formation where a cell may unequally localize cellular activities during division, thereby imparting disparity in the abundance of cell fate regulators in the daughter cells.