Abstract
In the past we have reported significant cognitive deficits in mice receiving 5-fluorouracil in combination with low-dose methotrexate. To explain such interactions, a pharmacokinetic study was designed. A sensitive bio-analytical method was therefore developed and validated for 5-fluorouracil and methotrexate in mouse plasma, brain and urine with liquid chromatography coupled to a single quadrupole mass spectrometer. Chromatographic separation was accomplished by Agilent® Zorbax® SB-C18 column, with isocratic elution (5 mM ammonium acetate and methanol, 70:30, %v/v) at a flow rate of 300 μL/min. The limit of quantitation for both drugs was 15.6 ng/mL (plasma and brain) and 78.1 ng/mL (urine), with interday and intraday precision and accuracy ≤15% and a total run time of 6 min. This bio-analytical method was used for the pharmacokinetic characterization of 5-fluorouracil and methotrexate in mouse plasma, brain and urine over a period of 24 h. This method allowed characterization of the brain concentrations of 5-fluorouracil over a period of 24 h.
Keywords: 5-fluorouracil, methotrexate, brain, LC-MS, pharmacokinetics
Introduction
5-Fluorouracil (5-FU) and methotrexate (MTX) are popular chemotherapeutic agents, and have been a common treatment option for breast cancer patients. However, recent studies suggest a possible role of such agents in cognitive deficits observed amongst patients (Ahles and Saykin, 2002, 2007). Pre-clinical in vitro and in vivo studies corroborate these observations (Dietrich et al., 2006, 2008; Fardell et al., 2010; Han et al., 2008). Our group has reported similar findings, wherein mice receiving a combination of 5-FU and MTX exhibited cognitive deficits (Foley et al., 2008). Such results demonstrating adverse neurological consequences for commonly used agents such as 5-FU and MTX have warranted a closer look at the possibility of pharmacokinetic (PK) interactions between 5-FU and MTX.
For 5-FU (Fig. 1) numerous bio-analytical methods, both quantitative and qualitative, have been reported in the past. Reverse-phase high-performance liquid chromatography (HPLC) has been commonly used with a variety of different detectors such UV–vis, fuorescence, NMR, MS and MS/MS (Heggie et al., 1987; Jarugula and Boudinot, 1996; Liu et al., 2010, 2012; Peer et al., 2012). Of all the methods available for 5-FU detection, HPLC methods with MS detectors are the most sensitive and reliable. Unfortunately most of these methods are fraught with shortcomings, such as complex sample extraction procedures (Joulia et al., 1997; Liu et al., 2012; Wang et al., 1998), complex chromatographic conditions (Pisano et al., 2005; Remaud et al., 2005), low sensitivity (Chu et al., 2003; van Kuilenburg et al., 2006) and the requirement for large sample volumes (Coe et al., 1996; Li et al., 2005; Liu et al., 2012; Loos et al., 1999; Peer et al., 2012). In addition, for 5-FU most reported studies use only plasma, while brain and urine matrices have not been evaluated (Büchel et al., 2013; Casale et al., 2002; Ciccolini et al., 2004; Joulia et al., 1997; Kosovec et al., 2008; Liu et al., 2010, 2012). Analytical methods determining 5-FU concentration in cerebrospinal fluid have been reported (Bourke et al., 1973) but were not very successful in tissues such as the brain (Bourke et al., 1973; Hao et al., 2004). For MTX (Fig. 1), a weak dicarboxylic acid, numerous analytical methods have been reported. HPLC methods with different detectors such as UV–vis, MS and MS/MS have been reported for MTX and its metabolites as well in different biological matrices with acceptable sensitivities (den Boer et al., 2012; Guo et al., 2007; Lobo and Balthasar, 2003).
Figure 1.
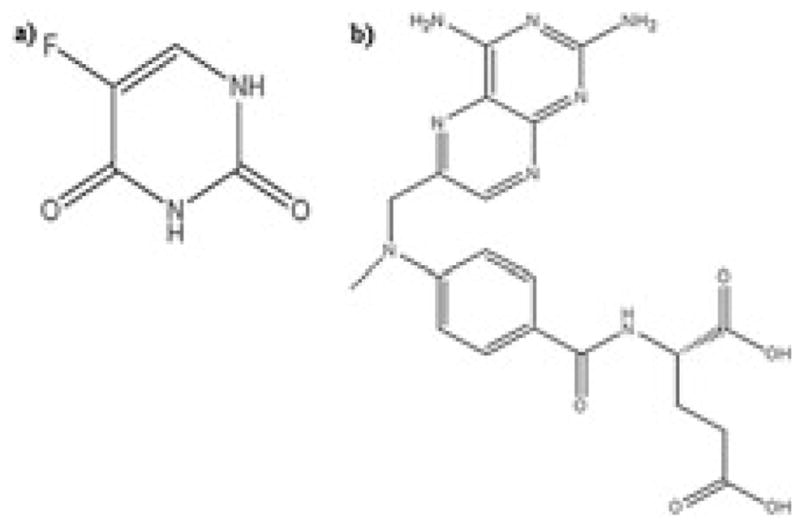
Chemical structures for (a) 5-fluorouracil (5-FU), molecular weight-130.08 Da; (b) methotrexate (MTX), molecular weight 454.44 Da.
However, there are very few sensitive analytical methods reported for a truly simultaneous determination of 5-FU and MTX in complex biological matrices such as the plasma, brain and urine. Sabatini et al. (2005) reported an analytical method for simultaneous quantification of 5-FU and MTX; however, the sample matrix used for quantification was Kleenex® tissue used for wiping surfaces. This sample matrix is not as complex as the biological matrices commonly used in PK studies. In this study we present the development and validation of a fast, sensitive and robust LC-MS method for 5-FU and MTX in biological matrices commonly utilized for preclinical as well as clinical PK and pharmacologic evaluation of drugs. The LC-MS parameters we used were the same for both 5-FU and MTX, therefore increasing the ease of analysis; however, we have used separate sample preparation techniques. This sensitive bio-analytical method was applied in the PK analysis for 5-FU and MTX in male Swiss–Webster mice (n = 3). This method allowed characterization of the brain concentrations of 5-FU over a period of 24 h, which has not been reported extensively in the past.
Experimental
Chemicals and reagents
5-FU was purchased from GeneraMedix Inc. (Ahmedabad, India); MTX was purchased from Hospira Inc. (Lake Forest, IL, USA). Aminopterin (AMP) and 5-bromouracil (5-BU) were purchased from Sigma-Aldrich Inc. (St Louis, MO, USA) to be used as internal standards (IS) for 5-FU and MTX, respectively. Ammonium acetate, glacial acetic acid and ethyl acetate (HPLC grade) were purchased from Fisher Scientific (Fairlawn, NJ, USA). HPLC-grade methanol was purchased from EMD chemicals Inc. (Gibbstown, NJ, USA). Deionized nanopure water was obtained from the nanopure de-ionization system (Barnstead/Thermolyne, Dubuque, IA, USA) located in the facility, and was used in all of the experiments. Blank mouse plasma (drug-free) was purchased from Lampire Biological laboratories (Pipersville, PA, USA). On receiving the blank plasma, aliquots of 10 mL were made and stored in a −20°C freezer. Batch number and date of receipt were noted.
Liquid chromatography and mass spectrometry parameters
The liquid chromatography (LC) system used was an Agilent 1100 series HPLC system (Agilent® Technologies, Santa Clara, CA, USA). For chromatographic separation an Agilent® Zorbax® SB-C18 (3.5 μm, 150 × 3 mm) analytical column coupled with a C18 guard cartridge (4 × 2.0 mm; Phenomenex, Torrance, CA, USA) was used. Columns were maintained at room temperature throughout the analysis. Sample volume injected into the system was 10 μL. Internal standards were 5-BU for 5-FU and AMP for MTX.
The mass spectrometry (MS) system used was an Agilent MSD SL-G1946D (Agilent® Technologies, Santa Clara, CA, USA). For determining appropriate MS parameters, flow injection analysis was performed with the drug solution at a concentration of 1 μg/mL in de-ionized nanopure water. The same mass spectrometer parameters were used for 5-FU and MTX. These parameters were applicable for all matrices (plasma, brain, urine) and were as follows: fragmentor voltage, 100 V; drying gas flow rate, 8 L/min; gas temperature, 250°C; nebulizer pressure, 40 psig; capillary voltage, 2500 V (±). Analysis for 5-FU and 5-BU was done in a negative ion mode with single ion monitoring (SIM) values of 129 and 189, respectively. For MTX and AMP, analysis was performed in a positive ionization mode with SIM values of 455 and 441, respectively. The MS detector described above has the capacity to quantify positive and negative ions simultaneously. The ionization source used for the method was electrospray ionization. All responses obtained were analyzed using the Agilent® ChemStation® software.
LC method development
With the parameters obtained for MS by flow injection analysis, we proceeded with developing appropriate LC parameters. Table 1 explains in detail various protocols evaluated for the determination of 5-FU and MTX in plasma, brain and urine samples.
Table 1.
Different methods tested for the simultaneous determination of 5-fluorouracil (5-FU) and methotrexate (MTX)
| Sample extraction procedures testeda,b | |||
|---|---|---|---|
| Protein precipitation | Double protein precipitation | Protein precipitation with LLE | LLE |
| A. Plasma + 3xc MeOH/3x ACN | A. Plasma + ammonium sulfate + MeOH–ACN | A. Plasma + 2x perchloric acid + 10x EA | A. Plasma + 10x dichloromethane |
| B. Plasma + TCA + GAA | B. Plasma + perchloric acid + MeOH/ACN | B. Plasma + 2x ammonium sulfate + 10x EA | B. Plasma + 3x MeOH–ACN + 10x EA |
| C. Plasma + ammonium sulfate | C. Plasma + 2x ammonium sulfate + n-propanol- dimethyl ether (8:2) | C. Plasma + 10x dichloromethane | |
| D. Plasma + perchloric acid | D. Plasma + 2x ammonium sulfate + GAA + 10x EA + back extraction with potassium hydroxide (0.5 M) | D. Plasma + 10x EA | |
| E. Plasma + 2x HCl + 10x EA–isopropanol (9:1) | E. Plasma + 3x MeOH–ACN + 10x EA | ||
| Mobile phases tested (pH = 4.0) | |||
| AA (1–20 mM; % v/v AA:MeOH), or AF (1–20mM; % v/v AF:ACN) | 40:60 | ||
| 50:50 | |||
| 60:40 | |||
| 70:30 | |||
| 90:10 | |||
| Reconstitution media tested | 50:50 (%v/v) buffer: organic | ||
| 100% organic | |||
| 100% aqueous | |||
| Final method selected based on: | Sample preparation | ||
| Sensitivity (LOQ), short run time, and ease of analysis | Protein precipitation – MTX (A) | ||
| LLE– 5FU (D) | |||
| Mobile phase | |||
| AA (5 mM, pH 4.0)–MeOH, 70:30% v/v | |||
| Reconstitution media | |||
| 50:50% (v/v) buffer – organic | |||
Abbreviations: MeOH, methanol; ACN, acetonitrile; TCA, trichloro acetic acid; GAA, glacial acetic acid; LLE, liquid–liquid extraction; EA, ethyl acetate; AA, ammonium acetate; AF, ammonium formate.
Each sample extraction procedure was individually performed with centrifugation speeds 4000, 8000 and 12,000 rpm.
Isocratic elution method was utilized for all combinations.
x, Plasma, brain, urine volume in microliters.
Preparation of stock solutions, calibration standards and quality controls
5-FU and MTX were commercially available as 50 mg/mL solutions in saline. For AMP, stock solutions were prepared in methanol with 4% DMSO at 200 μg/mL final concentration, and were stored in amber colored bottles at −20°C. For 5-BU, stock solutions were prepared in 100% methanol at 200 μg/mL final concentration, and were stored in amber colored bottles at −20°C. On the day of the experiment fresh stock solutions at 40 μg/mL concentration were prepared in de-ionized water for both the drugs. For the standard curve, calibration standards were prepared by adding appropriate aliquots from the stock solutions of 5-FU and MTX to the blank murine matrix (plasma, brain or urine), after which serial dilution was performed with the appropriate matrix to obtain a standard curve. The standard curve comprised seven nonzero concentrations ranging from 15.6 ng/mL to 1 μg/mL (plasma and brain) and from 78 ng/mL to 5μg/mL (urine). A working solution of AMP was prepared by diluting stock solutions with 100% methanol to make a final concentration of 15.6 ng/mL. A working solution of 5-BU was prepared by diluting stock solution with 100% ethyl acetate to make a final solution of 15.6 ng/mL. Separate stock solutions were prepared for quality control (QC) samples, and were used to validate the assay. For plasma and brain samples, three QC samples were chosen for each drug at the following concentrations to cover the entire standard curve range: 31.3 ng/mL (low), 125 ng/mL (medium) and 500 ng/mL (high). For urine samples three QC samples were chosen for each drug at the following concentrations: 156 ng/mL (low), 625 ng/mL (medium) and 2500 ng/mL (high).
Method validation
Calibration curve
Seven nonzero concentrations plus one blank sample comprised the calibration curve with concentrations 15.6, 31.3, 62.5, 125, 250, 500 and1000 ng/mL for plasma and brain samples and 78.1, 156.3, 312.5, 625, 1250, 2500 and 5000 ng/mL for urine samples. The standard curve was constructed by plotting the peak area ratios of drug and IS against theoretical concentrations of calibration standards. Linear regression analysis of the standard curve was performed using Graph Pad software (version 4.0), with a weighting factor of 1/x (where x = concentration). Back-calculation of standards and QCs was performed using the formula y = mx + c for evaluation of accuracy and precision on five separate occasions.
Accuracy and precision
Accuracy and precision of the given assay were determined for the three matrices containing known concentration of the drugs. Intraday accuracy and precision were determined by analyzing six replicates of QC samples on the same day. Interday accuracy and precision were determined by analyzing five replicates of QC samples, with the procedure performed on five separate occasions. At each concentration of the QC samples, precision was determined by calculating the percentage coefficient of variation, which was defined as:
Accuracy was determined by calculating the percentage relative error (RE), which was defined as: RE% = [(mean observed − theoretical)/ theoretical] × 100. The samples passed validation if the accuracy and precision at each QC concentration were ≤15% for all the conditions tested.
Extraction efficiency and matrix effect
Extraction efficiency and matrix effect were evaluated for plasma, brain and urine samples (n = 3) at concentrations of 31.3, 125 and 500 ng/mL for both 5FU and MTX. For this purpose, three sets of samples were prepared. In set 1, plasma, brain or urine samples were spiked with drug + IS prior to sample extraction process. In set 2, plasma, brain or urine samples were spiked with drug + IS post sample extraction. In set 3, drug and IS concentrations were prepared in a solution of 50:50% v/v ammonium acetate–methanol solution. Extraction efficiency (EE) was defined as:
Matrix Effect (ME) was calculated as:
Freeze–thaw stability
Freeze–thaw cycles for samples stored at −80°C were performed at 4 and 22 h for both 5-FU and MTX in plasma and brain (n = 2) at QC concentrations of 31.3, 125 and 500 ng/mL. Urine samples were analyzed immediately upon collection for the calculation of fe. Therefore freeze–thaw stability in urine was not tested. Relative error was calculated as:
Application to PK study
Male Swiss–Webster mice (5–6 weeks old; 25–30 g) were used for all PK studies. All study protocols were approved by the Institution of Animal Care and Use Committee (Temple University, Philadelphia, PA, USA). Animals were housed in well-ventilated cages, exposed to a regular light–dark cycle of 12 h each. Food and water were available ad-libitum.
Both 5-FU and MTX were available as 50 mg/mL solutions for injection. Suitable dilutions were made in sterile saline on the day of the study. A single dose of 75 mg/kg for 5-FU or 32 mg/kg for MTX was administered via intravenous (i.v.) bolus injection through the lateral tail vein using a 0.5 mL insulin syringe (EXELINT® International Co., Los Angeles, CA, USA) with a 29½ gauge needle.
A serial sacrifice sampling protocol was followed for the collection of blood and tissues. Under anesthesia, blood samples were collected in heparinized tubes from the inferior vena cava of mice at time points 5, 10, 15, 30, 45, 60 min and 2, 4, 8, 16, and 24 h. Plasma samples were obtained by collecting the supernatant obtained by centrifuging blood samples at 12,000 rpm for three min at 4°C. For collection of brain tissue samples, animals were sacrificed by cervical dislocation under anesthesia. Collected samples were cleaned, weighed and stored in −80°C until further analysis. For urine collection, animals were placed in individual metabolic cages (Nalgene; Braintree Scientific Inc., Braintree, MA, USA) that had discrete collection counters for feces and urine. Metabolic cages were inspected frequently, on average once every hour. Any urine sample present in the collection counter was removed and placed in a vial assigned specifically for the cumulative collection of urine samples. These samples were obtained over a period of 24 h, and each collected sample was placed immediately at −20°C until analysis.
Noncompartmental analysis (NCA) for 5-FU and MTX was performed to obtain primary and secondary PK parameters. Average (n = 3) plasma and tissue concentration data were used for the NCA performed by WinNonlin® version 6.0 (Phoenix). Area under the curve (AUC0–∞) and area under the first moment curve (AUMC0–∞) were obtained by integrating concentration–time (C–t) data in plasma and brain from time zero to infinity. Systemic clearance was defined as CLs = dose/AUC0–∞. For the parameters mean residence time (MRT) and volume of distribution at steady state (Vss), estimates were obtained using the statistical moment theory (Kong and Jusko, 1988), where MRT = AUMC0–∞/AUC0–∞, Vss = CLs × MRT. Cmax and Tmax represent maximal concentration and the time to reach maximal concentration respectively. The elimination rate constant (kel) was calculated as kel = 1/MRT, and the half-life (t1/2) was calculated as t1/2 = 0.693/kel. The fraction of drug eliminated unchanged in urine (fe) was calculated as amount excreted unchanged in urine (0–24 h)/dose amount administered.
Results and discussion
LC method development
Several methods were examined to establish a sensitive, robust and simple bio-analytical method for 5-FU and MTX (Table 1). Of all the methods tested, the most important criteria for selecting the final LC-MS method were sensitivity, short run time and ease of analysis. Machine parameters chosen for LC and MS as reported here and also in the Experimental section above, were amenable for both 5-FU and MTX. The mobile phase for both 5-FU and MTX consisted of ammonium acetate (5 mM, pH 4) and 100% methanol (70:30%, v/v) with an isocratic elution at a flow rate of 300 μL/min. Retention times for the analytes were: 2.6 min (5-FU), 3.2 min (5-BU), 5.7 min (MTX) and 3.5 min (AMP). Representative chromatograms for 5-FU and MTX are shown in Figs 3 and 4.
Figure 3.
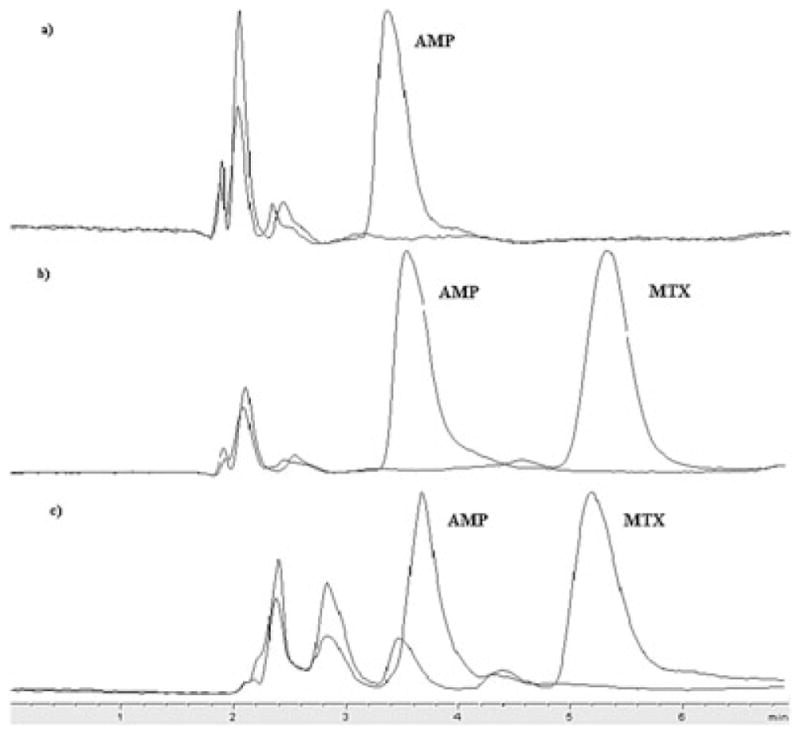
Representative chromatograms for MTX and internal standard aminopterin (AMP) in (a) blank plasma (only AMP is present); (b) plasma spiked with LOQ concentration; and (c) in vivo collected sample for MTX.
Figure 4.
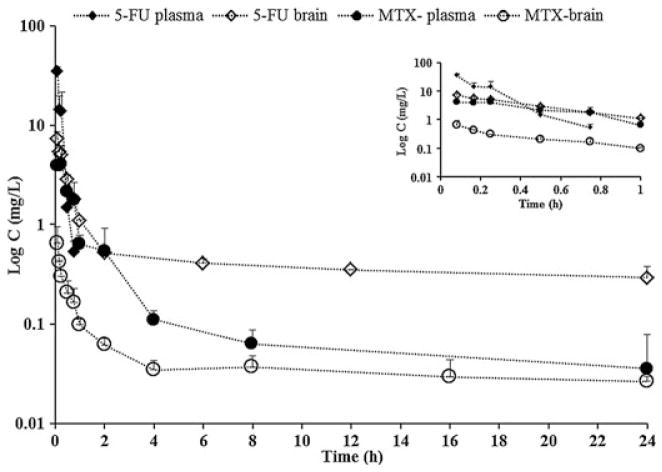
Concentration–time profiles for 5-FU and MTX in mouse plasma and brain. All values are represented as means ± SD, with n = 3. Inset depicts concentration–time profiles for 5-FU and MTX in mouse plasma and brain from 0 to 1 h.
In the past, 5-FU analytical methods have commonly involved HPLC methods with UV detectors (Coe et al., 1996; Heggie et al., 1987; Jarugula and Boudinot, 1996; Joulia et al., 1997). Recently MS detectors have been used, with reported limit of quantitation (LOQ) values of 130 ng/mL (van Kuilenburg et al., 2006), 10 ng/mL (Kosovec et al. 2008; Woo et al. 2008) and 5 ng/mL (Licea-Perez et al., 2009), which are comparable to our reported value (15.6 ng/mL). However these methods have some drawbacks, such as complex derivitization procedures for sample preparation (Licea-Perez et al., 2007; Liu et al., 2010), absence of a validated method in matrices other than plasma (Kosovec et al., 2008) and longer run times (van Kuilenburg et al., 2006). Similarly for MTX, HPLC methods with UV (Aboleneen et al., 1996; Sartori et al., 2008; Sparreboom et al., 1999) or fuorescence detectors (Lobo and Balthasar, 2003) have been reported. Recent studies have reported LOQ values of 3.9 ng/mL (Blakeley et al., 2009), which are comparable to our LOQ of 15.6 ng/mL; however, concentrations as low as 3.9 ng/mL are rarely observed for MTX in a PK study spread over 24 h (den Boer et al., 2012). Also, there are very few analytical methods that deal with a truly simultaneous determination of 5-FU and MTX. In this study we applied discrete sample preparation methods for the two drugs. However, we established common LC-MS machine parameters for both 5-FU and MTX in complex biological matrices such as the plasma, brain and urine. In doing so, we have improved the efficiency of analysis of these two drugs together as opposed to developing completely separate analytical methods as was previously performed.
Sample preparation
Of all the sample preparation protocols that were tested (Table 1), the following sample preparation methods provided the best results analytically – in terms of sensitivity, short run time and ease of analysis. For 5-FU, to 50 μL (plasma) or 20 μL (brain or urine samples) plus drug, a 10x volume of ethyl acetate + IS (EA + IS) solution was added. This was then placed in a shaker for 10 min, after which it was centrifuged at 12,000 rpm for 10 min at 4°C. The upper layer was removed completely and collected in a fresh Eppendorf tube. This process was repeated by adding a 10x volume of fresh EA + IS solution to the remaining plasma or brain tissue sample. The upper layer was removed completely and collected again. This collected mixture was dried under N2 for 15 min. The dried residue was reconstituted with 50 μL of mobile phase (plasma sample) or 20 μL of mobile phase (brain or urine samples). Ten microliters of this solution was injected in the LC/MS system for analysis.
For MTX, to 50 μL (plasma) or 20 μL (brain, or urine samples) plus drug, a 3x volume of methanol + IS (M + IS) solution was added. This was then vortexed for 10 s and later centrifuged at 12,000 rpm at 4°C for 10 min. The supernatant was collected and dried under N2 for 30 min. Dried residue was reconstituted with 50 μL of mobile phase (plasma sample) or 20 μL of mobile phase (brain or urine samples). This solution (10 μL) was injected in the LC/MS systems for analysis.
Methods that have reported a truly simultaneous determination of 5-FU and MTX have done so in ‘neat samples’ and not complex matrices such as plasma, brain or urine (Sabatini et al., 2005). Although we report two separate sample preparation methods for 5-FU and MTX, machine parameters for LC and MS were the same for both 5-FU and MTX in all matrices. Having common LC and MS machine parameters makes the analytical method consistent across samples and also more efficient and user friendly. Combination studies for 5-FU and MTX performed in the past reported distinctly separate analytical methods for each drug (Batey et al., 2002; de Bruijn et al., 1987; Moore et al., 1994), unlike our method, where only the sample preparation is different for the two drugs. In addition our analytical method is sensitive (LOQ 15.6 ng/mL), has simple sample extraction methods (protein precipitation/liquid–liquid extraction), a short run time (6 min) and uncomplicated liquid chromatography conditions (isocratic elution), and is robust (Table 2).
Table 2.
Method validation for 5-FU and MTX in mouse plasma, brain and urine
| Matrix | Interday | Intraday | |||||
|---|---|---|---|---|---|---|---|
| Theoretical (ng/mL) | Observed (ng/mL) | CV (%)a | RE (%)b | Observed (ng/mL) | CV (%)a | RE (%)b | |
| 5-Fluorouracil | |||||||
| Plasma | 15.6 | 17.2 ± 3.3 | 19.1 | 9.3 | 14.8 ± 1.7 | 11.5 | −5.5 |
| 31.3 | 33.9 ± 4.5 | 13.3 | 8.3 | 31.8 ± 1.9 | 5.9 | 1.8 | |
| 125 | 141.9 ± 21 | 14.8 | 13.5 | 139.7 ± 13.8 | 9.9 | 11.8 | |
| 500 | 501.2 ± 74.1 | 14.8 | 0.2 | 493.3 ± 31.6 | 6.4 | −1.3 | |
| Brain | 15.6 | 15.4 ± 2.4 | 15.6 | −1.2 | 14.5 ± 1.5 | 10.1 | −6.8 |
| 31.3 | 29.5 ± 2.5 | 8.5 | −6.0 | 29.9 ± 2.3 | 7.7 | −4.2 | |
| 125 | 125.8 ± 14.5 | 11.5 | 0.7 | 128.8 ± 17.9 | 13.9 | 2.9 | |
| 500 | 514.7 ± 26.2 | 5.1 | 2.9 | 510.9 ± 26.1 | 5.1 | 2.1 | |
| Urine | 78.1 | 71.4 ± 11 | 15.6 | −9.4 | 72.6 ± 1.4 | 19.1 | −4.7 |
| 156.3 | 143.7 ± 16.5 | 11.5 | −8.7 | 157.2 ± 23 | 14.6 | 0.7 | |
| 625 | 585.9 ± 61.5 | 10.5 | −6.7 | 568.9 ± 33 | 5.8 | −9.9 | |
| 2500 | 2637.5 ± 308.6 | 11.7 | 5.2 | 2537.3 ± 294.3 | 11.6 | 1.5 | |
| Methotrexate | |||||||
| Plasma | 15.6 | 13.9 ± 1.5 | 11.0 | −12.6 | 14.0 ± 1.6 | 11.6 | −11.9 |
| 31.3 | 36.7 ± 5.5 | 15.1 | 11.5 | 33.8 ± 4.7 | 13.8 | 7.6 | |
| 125 | 124.1 ± 14.5 | 11.7 | −0.8 | 119.2 ± 14.2 | 11.9 | −4.9 | |
| 500 | 505.2 ± 51.5 | 10.2 | 1.0 | 474.4 ± 39 | 8.2 | −5.4 | |
| Brain | 15.6 | 15.4 ± 1.0 | 6.7 | 1.9 | 16.9 ± 2.5 | 14.6 | 7.6 |
| 31.3 | 29.5 ± 2.7 | 9.4 | −10.6 | 30.1 ± 1.4 | 4.6 | −3.9 | |
| 125 | 123.1 ± 8.0 | 6.5 | −1.5 | 130.0 ± 7.5 | 5.8 | 3.8 | |
| 500 | 512.2 ± 6.1 | 1.2 | 2.4 | 503.3 ± 40.3 | 8.0 | 0.7 | |
| Urine | 78.1 | 71.6 ± 10.7 | 14.9 | −9.0 | 78.7 ± 14.2 | 18.1 | 0.8 |
| 156.3 | 167.8 ± 22.7 | 13.5 | 6.9 | 142.7 ± 20 | 14.0 | −9.4 | |
| 625 | 565.3 ± 55.4 | 9.8 | −10.1 | 629.6 ± 98.2 | 15.6 | 0.7 | |
| 2500 | 2513.3 ± 83 | 3.3 | 0.51 | 2446.4 ± 203.1 | 8.3 | −2.1 | |
Precision (CV %) = (standard deviation/mean) × 100.
Bias (RE %) = [(mean observed − theoretical value)/theoretical value] × 100.
Concentrations measured are reported as means ± SD; n = 5 for interday and n = 6 for intraday.
Calibration curve
Seven nonzero concentrations over a range of 15.6 ng/mL to 1μg/mL for mouse plasma and brain and 78.1 ng/mL to 5μg/mL in mouse urine were used to establish a calibration curve. The standard curve was constructed by plotting the peak area ratios of drug and IS against theoretical concentrations of calibration standards and fitted to the linear equation: y = mx + c using a weighing factor (1/x). For five replicates, average r2 was ≥0.99. Accuracy and precision of back-calculated concentrations for all QCs were ≤15%. The LOQ represented by the lowest concentration on the standard curve demonstrated appropriate accuracy and precision of ≤20% (Table 2).
Accuracy and precision
Method validation was performed according to the guidelines provided by the US Food and Drug Administration (Shah et al., 1991). The LOQ was defined as the lowest concentration on the standard curve for which the accuracy and precision was ≤20% on five separate occasions. The LOQ for 5-FU and MTX in plasma and brain was 15.6 ng/mL and LOQ for 5-FU and MTX was 78 ng/mL in urine. The interday and intraday accuracy and precision for each QC was ≤15%. Assay validation for both 5-FU and MTX is reported in Table 1. For 5-FU very few studies have reported an analytical method for determining concentrations in brain; however, when a single dose of 50 mg/kg of 5-FU was administered in vivo, the analytical method was not sensitive enough to quantify 5-FU concentrations over time in brain tissue (Jin et al., 2005; Pisano et al., 2005). In contrast, we applied our analytical method in describing the PK profile of a single bolus dose of 75 mg/kg 5-FU for 24 h in brain. Chromatograms of LOQ in plasma for both 5-FU and MTX are shown in Figs 2 and 3.
Figure 2.
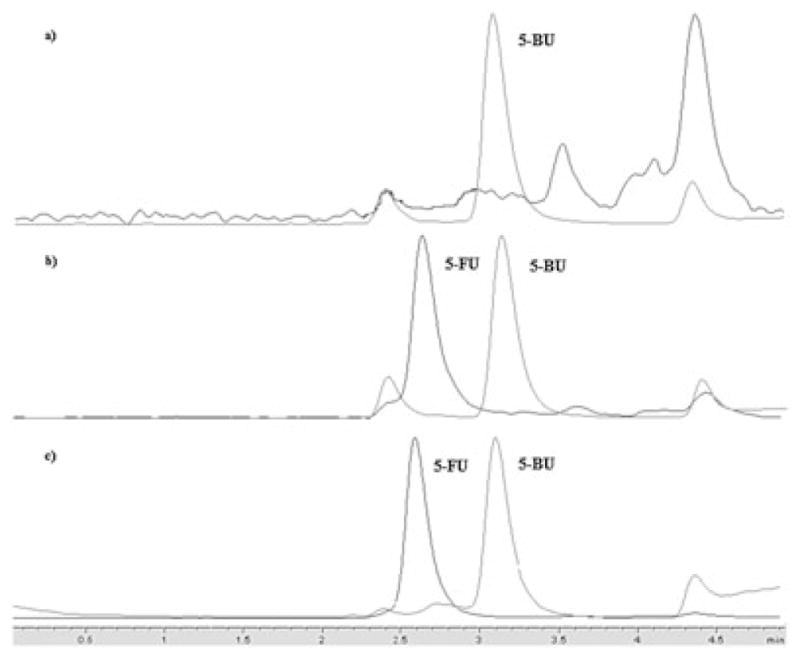
Representative chromatograms for 5-FU and internal standard 5-bromouracil (5-BU) in (a) blank plasma (only 5-BU is present); (b) plasma spiked with LOQ concentration; and (c) in vivo collected sample for 5-FU.
Extraction efficiency and matrix effect
Extraction efficiencies as well as matrix effect for 5-FU and MTX in plasma, brain and urine are reported in Table 3. In all matrices, 5-FU exhibited high extraction efficiency (~90%), while MTX had moderate extraction efficiency (~40%). Matrix effects observed for MTX were moderate in all three matrices (~30%), while for 5-FU matrix effects were high (~70%).
Table 3.
Extraction efficiency and matrix effect for 5-FU and MTX in mouse plasma, brain and urine
| Biological matrix | Added (ng/mL) | Extraction efficiencya (% mean, n = 3) | Matrix effectb (% mean; n = 3) | ||
|---|---|---|---|---|---|
| MTX | 5-FU | MTX | 5-FU | ||
| Plasma | 31.3 | 53.5 ± 15.8 | 65.4 ± 20.2 | 42.3 ± 8.1 | 8.9 ± 10.4 |
| 125 | 58.1 ± 20.9 | 53.3 ± 29.0 | 31.3 ± 7.7 | 18.6 ± 23.5 | |
| 500 | 36.8 ± 4.9 | 97.9 ± 20.2 | 16.5 ± 8.0 | 78.6 ± 0.9 | |
| Brain | 31.3 | 24.1 ± 4.7 | 114.8 ± 16.9 | 17.0 ± 6.9 | 77.2 ± 5.3 |
| 125 | 43.5 ± 9.2 | 108.4 ± 13.9 | 21.0 ± 11.1 | 45.6 ± 9.1 | |
| 500 | 44.3 ± 4.6 | 107.4 ± 13.1 | 20.6 ± 15.0 | 69.8 ± 1.2 | |
| Urine | 31.3 | 31.7 ± 4.6 | 114.2 ± 1.5 | 37.0 ± 7.3 | 75.2 ± 20.2 |
| 125 | 43.5 ± 11.2 | 76.1 ± 19.2 | 21.0 ± 17.0 | 35.2 ± 5.0 | |
| 500 | 44.2 ± 5.1 | 83.5 ± 48.2 | 20.6 ± 10.0 | 49.5 ± 16.7 | |
All values are represented as means ± SD; n = 3.
Extraction efficiency = (set1/set2) × 100.
Matrix effect = 100 − [(set2/set3) × 100]. Sets 1–3 are defined in the Experimental section in the text.
Ion suppression as a result of matrix effect is a common drawback of MS and MSn detection methods. The mechanisms for such matrix effects are not fully understood; however, ion suppression is most commonly attributed to the presence of endogenous compounds, surface tension, pH, polymers from plastic tubes used for sample preparation, ionization source, basicity, high concentration of analyte or elution of analyte of interest in the solvent front (Jessome and Volmer, 2006; King et al., 2000). The presence of ion suppression as a result of matrix effect may be detrimental to the analytical method in terms of accuracy, precision and even detection of analyte (Bakhtiar and Majumdar, 2007; Cullum et al., 2004; Jessome and Volmer, 2006). Although the analytical method described for 5-FU exhibited significant matrix effect (Table 3), it did not affect the analytical capability of this method as shown in the method validation report (Table 2). Also, this method did not hinder the detection of analytes, as we could detect drug levels LOQ in brain for 24 h.
Freeze–thaw stability
Both 5-FU and MTX were tested for stability in plasma and brain over two freeze–thaw cycles as described under Experimental. Relative errors for 5-FU and MTX for freeze thaw cycles were each ~20%, indicating acceptable stability for subsequent study conditions. Results are shown in Table 4.
Table 4.
Freeze–thaw stability for 5-FU and MTX in mouse plasma and brain samples
| Biological matrix | Theoretical (ng/mL) | RE%a (4 h) | RE% (22 h) | ||
|---|---|---|---|---|---|
| MTX | 5-FU | MTX | 5-FU | ||
| Plasma | 31.3 | −4.0 | −11.2 | −2.0 | −26.6 |
| 125 | −16.2 | −25.1 | 3.0 | −5.6 | |
| 500 | −0.7 | −10.5 | −6.4 | −3.0 | |
| Brain | 31.3 | 15.9 | −13.1 | 7.2 | 21.3 |
| 125 | 21.2 | −8.4 | 1.5 | −7.1 | |
| 500 | −15.0 | −0.3 | −3.9 | −1.3 | |
Percentage relative error (RE %) = [(mean observed − theoretical value)/theoretical value] × 100.
Application to PK study
We have successfully applied our validated bio-analytical method to a PK study determining plasma and brain C–t profiles and fe for 5-FU and MTX, when administered as a single i.v. bolus dose. Plasma and brain C–t profiles of animals receiving single i.v. bolus doses of 75 mg/kg 5-FU and 32 mg/kg MTX are shown in Fig. 4. PK parameters obtained by noncompartmental analysis of plasma and brain C–t data are shown in Table 5. For 5-FU, C–t profile was detectable in the brain for up to 24 h, whereas in plasma drug levels fell below detectable limits of the machine after 45 min. 5-FU showed a monophasic decline over a period of 45 min, whereas the C–t profile in the brain was found to be biphasic with a Tmax of 5 min, indicating no lag time for 5-FU in brain. Plasma t1/2 for 5-FU was found to be 5.2 ± 0.9 min, which is similar to the values reported previously (Yi et al., 2010). Plasma CLs and Vss values reported for 5-FU as shown in Table 5 in our study are similar to values reported previously in mice (Jin et al., 2005), rats (Jarugula et al., 1997; Pinedo and Peters, 1988), dogs (Kuan et al., 1998) and humans (Diasio and Harris, 1989). In brain 5-FU has a kel of 0.00035 min−1, which indicates very slow elimination of the drug. Such a prolonged retention for 5-FU has been reported in murine tumors previously, suggesting that the hydrophilic drug may have a tendency to be trapped in more lipophilic tissues (Peters et al., 1993).
Table 5.
Noncompartmental pharmacokinetic parameters for single i.v. bolus dose of 75 mg/kg 5-FU or i.v. bolus dose of 32 mg/kg MTX
| PK parameters (units) | 5 FU (75 mg/kg) | MTX (32 mg/kg) |
|---|---|---|
| AUCplasma (0–∞) (min mg/L) | 631.0 ± 78.9 | 300.1 ± 65 |
| Cmax (mg/L) | 34.8 ± 4.1 | 4.3 ± 0.05 |
| Tmax (min) | 5 | 5 |
| CLs (L/min/kg) | 0.12 ± 0.03 | 0.11 ± 0.03 |
| AUCbrain (0–24) (min mg/L) | 748.8 ± 122 | 66.3 ± 12.9 |
| Vss (L/kg) | 0.9 ± 03 | 23 ± 5.1 |
| t1/2 (min) | 5.2 ± 0.9 | 152.5 ± 63.7 |
| fe | 0.08 ± 0.01 | 0.2 ± 0.06 |
In plasma MTX has a biphasic decline. Plasma t1/2 was 152.5±63.7 min, and is in with accordance values reported previously (Olsen, 1991). CLs and Vss values reported for MTX as shown in Table 5 in our study are were similar to values reported previously in mice (Wang et al., 2011), rats (Fahrig et al., 1989; Miglioli et al., 1985) and humans (Jolivet et al., 1983). Brain C–t profile for MTX also showed biphasic decline, with a Tmax at 5 min, indicating no lag time for MTX detection in brain. In brain, kel for MTX was 0.001 min−1. MTX is also known to exhibit nonlinear kinetics attributed to saturable renal excretory mechanisms and enterohepatic re-cycling (Hendel and Brodthagen, 1984; Hendel and Nyfors, 1984; Olsen, 1991).
PK studies for 5-FU in the brain have not been reported extensively. Characterization of the profile of 5-FU in the brain opens up new avenues for the study of 5-FU-related neurotoxicity with or without other drugs such as MTX. Cytotoxicity synergism between 5-FU and MTX has been reported in the past (Bertino et al., 1983; Cadman et al., 1981; Fernandes and Bertino, 1980; Herrmann et al., 1984; Katzir et al., 2000). Given the sensitivity of the analytical method described above for both 5-FU and MTX in plasma and brain, we plan to explore this synergism between 5-FU and MTX by performing a PK study to delineate a possible cause for the reported neurotoxic effects by our group (Foley et al., 2008). In our future studies we plan to perform PK studies for 5-FU and MTX at different dose levels administered individually and in combination, via multiple dosing routes. Such a drugdrug interaction (DDI) study will be extremely crucial in providing a novel perspective to explain increased cognitive deficits observed in mice receiving a combination of 5-FU and MTX.
Conclusions
In conclusion, a rapid and sensitive bio-analytical method for the simultaneous determination 5-FU and MTX was developed and validated in mouse plasma, brain and urine. The above bioanalytical method was used for quantitating 5-FU and MTX in plasma and brain over time for up to 24 h. Also, this method will be used in the future for analyzing possible DDIs between 5-FU and MTX in mice.
Acknowledgments
The work was partially supported by award number R01CA129092 from the National Institutes of Health to E.A.W.
Abbreviations used
- 5-FU
5-fluorouracil
- AUC
area under curve
- AUMC
area under the first moment curve
- CS
calibration standards
- EE
extraction efficiency
- fe
fraction of drug eliminated unchanged in urine
- ME
matrix effect
- MRT
mean residence time
- MTX
methotrexate
- NCA
non compartmental analysis
- PK
pharmacokinetics
References
- Aboleneen H, Simpson J, Backes D. Determination of methotrexate in serum by high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl. 1996;681:317–322. doi: 10.1016/0378-4347(95)00580-3. [DOI] [PubMed] [Google Scholar]
- Ahles TA, Saykin AJ. Breast cancer chemotherapy-related cognitive dysfunction. Clin Breast Cancer. 2002;3(suppl 3):S84–90. doi: 10.3816/cbc.2002.s.018. [DOI] [PubMed] [Google Scholar]
- Ahles TA, Saykin AJ. Candidate mechanisms for chemotherapy-induced cognitive changes. Nat Rev Cancer. 2007;7:192–201. doi: 10.1038/nrc2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakhtiar R, Majumdar TK. Tracking problems and possible solutions in the quantitative determination of small molecule drugs and metabolites in biological fluids using liquid chromatography–mass spectrometry. J Pharmacol Toxicol Methods. 2007;55:262–278. doi: 10.1016/j.vascn.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Batey MA, Wright JG, Azzabi A, Newell DR, Lind MJ, Calvert AH, Boddy AV. Population pharmacokinetics of adjuvant cyclophosphamide, methotrexate and 5-fluorouracil (CMF) Eur J Cancer. 2002;38:1081–1089. doi: 10.1016/s0959-8049(02)00024-2. [DOI] [PubMed] [Google Scholar]
- Bertino JR, Mini E, Fernandes DJ. Sequential methotrexate and 5-fluorouracil: mechanisms of synergy. Semin Oncol. 1983;10:2–5. [PubMed] [Google Scholar]
- Blakeley JO, Olson J, Grossman SA, He X, Weingart J, Supko JG New Approaches to Brain Tumor Therapy (NABTT) Consortium. Effect of blood brain barrier permeability in recurrent high grade gliomas on the intratumoral pharmacokinetics of methotrexate: a microdialysis study. J Neurooncol. 2009;91:51–58. doi: 10.1007/s11060-008-9678-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourke RS, West CR, Chheda G, Tower DB. Kinetics of entry and distribution of 5-fluorouracil in cerebrospinal fluid and brain following intravenous injection in a primate. Cancer Res. 1973;33:1735–1746. [PubMed] [Google Scholar]
- Büchel B, Rhyn P, Schürch S, Bühr C, Amstutz UR, Largiadèr C. LC-MS/MS method for simultaneous analysis of uracil, 5,6-dihydrouracil, 5-fluorouracil and 5-fluoro-5,6-dihydrouracil in human plasma for therapeutic drug monitoring and toxicity prediction in cancer patients. Biomedical Chromatography. 2013;27:7–16. doi: 10.1002/bmc.2741. [DOI] [PubMed] [Google Scholar]
- Cadman E, Heimer R, Benz C. The influence of methotrexate pretreatment on 5-fluorouracil metabolism in L1210 cells. J Biol Chem. 1981;256:1695–1704. [PubMed] [Google Scholar]
- Casale F, Canaparo R, Muntoni E, Serpe L, Zara GP, Della Pepa C, Berno E, Costa M, Eandi M. Simultaneous HPLC determination of 5-fluorouracil and its metabolites in plasma of cancer patients. Biomed Chromatogr. 2002;16:446–52. doi: 10.1002/bmc.181. [DOI] [PubMed] [Google Scholar]
- Chu D, Gu J, Liu W, Paul Fawcett J, Dong Q. Sensitive liquid chromatographic assay for the simultaneous determination of 5-fluorouracil and its prodrug, tegafur, in beagle dog plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;795:377–382. doi: 10.1016/s1570-0232(03)00571-3. [DOI] [PubMed] [Google Scholar]
- Ciccolini J, Mercier C, Blachon MF, Favre R, Durand A, Lacarelle B. A simple and rapid high-performance liquid chromatographic (HPLC) method for 5-fluorouracil (5-FU) assay in plasma and possible detection of patients with impaired dihydropyrimidine dehydrogenase (DPD) activity. J Clin Pharm Ther. 2004;29:307–315. doi: 10.1111/j.1365-2710.2004.00569.x. [DOI] [PubMed] [Google Scholar]
- Coe RA, Earl RA, Johnson TC, Lee JW. Determination of 5-fluorouracil in human plasma by a simple and sensitive reversed-phase HPLC method. J Pharm Biomed Anal. 1996;14:1733–1741. doi: 10.1016/0731-7085(96)01832-8. [DOI] [PubMed] [Google Scholar]
- Cullum N, Meng CK, Zavitsanos P. Effect of Sample Matrix on Suppression of Ionization in Water Samples Using LC-ESI-MS. Agilent Technologies; Santa Clara, CA: 2004. pp. 1–10. [Google Scholar]
- de Bruijn EA, Driessen O, Leeflang P, van Strijen E, van den Bosch N, Hermans J. Interactions of methotrexate and cyclophosphamide with the pharmacokinetics of 5-fluorouracil in an animal model. Cancer Treat Rep. 1987;71:1267–1269. [PubMed] [Google Scholar]
- den Boer E, Heil SG, van Zelst BD, Meesters RJ, Koch BC, Te Winkel ML, van den Heuvel-Eibrink MM, Luider TM, de Jonge R. A U-HPLC-ESI-MS/ MS-based stable isotope dilution method for the detection and quantitation of methotrexate in plasma. Ther Drug Monit. 2012;34:432–439. doi: 10.1097/FTD.0b013e31825bb368. [DOI] [PubMed] [Google Scholar]
- Diasio RB, Harris BE. Clinical pharmacology of 5-fluorouracil. Clin Pharmacokinet. 1989;16:215–237. doi: 10.2165/00003088-198916040-00002. [DOI] [PubMed] [Google Scholar]
- Dietrich J, Han R, Yang Y, Mayer-Pröschel M, Noble M. CNS progenitor cells and oligodendrocytes are targets of chemotherapeutic agents in vitro and in vivo. J Biol. 2006;5:22. doi: 10.1186/jbiol50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich J, Monje M, Wefel J, Meyers C. Clinical patterns and biological correlates of cognitive dysfunction associated with cancer therapy. Oncologist. 2008;13:1285–1295. doi: 10.1634/theoncologist.2008-0130. [DOI] [PubMed] [Google Scholar]
- Fahrig L, Brasch H, Iven H. Pharmacokinetics of methotrexate (MTX) and 7-hydroxymethotrexate (7-OH-MTX) in rats and evidence for the metabolism of MTX to 7-OH-MTX. Cancer Chemother Pharmacol. 1989;23:156–160. doi: 10.1007/BF00267947. [DOI] [PubMed] [Google Scholar]
- Fardell JE, Vardy J, Logge W, Johnston I. Single high dose treatment with methotrexate causes long-lasting cognitive dysfunction in laboratory rodents. Pharmacol Biochem Behav. 2010;97:333–339. doi: 10.1016/j.pbb.2010.08.019. [DOI] [PubMed] [Google Scholar]
- Fernandes DJ, Bertino JR. 5-Fluorouracil-methotrexate synergy: enhancement of 5-fluorodeoxyridylate binding to thymidylate synthase by dihydropteroylpolyglutamates. Proc Natl Acad Sci USA. 1980;77:5663–5667. doi: 10.1073/pnas.77.10.5663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley JJ, Raffa RB, Walker EA. Effects of chemotherapeutic agents 5-fluorouracil and methotrexate alone and combined in a mouse model of learning and memory. Psychopharmacology (Berlin) 2008;199:527–538. doi: 10.1007/s00213-008-1175-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo P, Wang X, Liu L, Belinsky MG, Kruh GD, Gallo JM. Determination of methotrexate and its major metabolite 7-hydroxymethotrexate in mouse plasma and brain tissue by liquid chromatography–tandem mass spectrometry. J Pharm Biomed Anal. 2007;43:1789–1795. doi: 10.1016/j.jpba.2006.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han R, Yang YM, Dietrich J, Luebke A, Mayer-Pröschel M, Noble M. Systemic 5-fluorouracil treatment causes a syndrome of delayed myelin destruction in the central nervous system. J Biol. 2008;7:12. doi: 10.1186/jbiol69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao X, Cheng G, Zou M, Sun J, Cui F. Organ distribution of 5-fluorouracil loaded gelatine microspheres in mice. Pharmazie. 2004;59:709–712. [PubMed] [Google Scholar]
- Heggie GD, Sommadossi JP, Cross DS, Huster WJ, Diasio RB. Clinical pharmacokinetics of 5-fluorouracil and its metabolites in plasma, urine, and bile. Cancer Res. 1987;47:2203–2206. [PubMed] [Google Scholar]
- Hendel J, Brodthagen H. Entero-hepatic cycling of methotrexate estimated by use of the D-isomer as a reference marker. Eur J Clin Pharmacol. 1984;26:103–107. doi: 10.1007/BF00546716. [DOI] [PubMed] [Google Scholar]
- Hendel J, Nyfors A. Nonlinear renal elimination kinetics of methotrexate due to saturation of renal tubular reabsorption. Eur J Clin Pharmacol. 1984;26:121–124. doi: 10.1007/BF00546719. [DOI] [PubMed] [Google Scholar]
- Herrmann R, Manegold C, Schroeder M, Tigges FJ, Bartsch H, Jungi F, Fritze D. Sequential methotrexate and 5-FU in breast cancer resistant to the conventional application of these drugs. Cancer Treat Rep. 1984;68:1279–1281. [PubMed] [Google Scholar]
- Jarugula VR, Boudinot FD. High-performance liquid chromatographic determination of 5-fluorouracil and its prodrugs, tegafur and 4-deoxy-5-fluorouracil, in rat plasma. J Chromatogr B Biomed Sci Appl. 1996;677:199–203. doi: 10.1016/0378-4347(95)00438-6. [DOI] [PubMed] [Google Scholar]
- Jarugula VR, Lam SS, Boudinot FD. Nonlinear pharmacokinetics of 5-fluorouracil in rats. J Pharm Sci. 1997;86:756–758. doi: 10.1021/js960451a. [DOI] [PubMed] [Google Scholar]
- Jessome LL, Volmer DA. Ion suppression: a major concern in mass spectrometry. LCGC North America. 2006;24:8. [Google Scholar]
- Jin Y, Li J, Rong LF, Lü XW, Huang Y, Xu SY. Pharmacokinetics and tissue distribution of 5-fluorouracil encapsulated by galactosylceramide liposomes in mice. Acta Pharmacol Sin. 2005;26:250–256. doi: 10.1111/j.1745-7254.2005.00530.x. [DOI] [PubMed] [Google Scholar]
- Jolivet J, Cowan KH, Curt GA, Clendeninn NJ, Chabner BA. The pharmacology and clinical use of methotrexate. N Engl J Med. 1983;309:1094–1104. doi: 10.1056/NEJM198311033091805. [DOI] [PubMed] [Google Scholar]
- Joulia JM, Pinguet F, Grosse PY, Astre C, Bressolle F. Determination of 5-fluorouracil and its main metabolites in plasma by high-performance liquid chromatography: application to a pharmacokinetic study. J Chromatogr B Biomed Sci Appl. 1997;692:427–435. doi: 10.1016/s0378-4347(96)00518-x. [DOI] [PubMed] [Google Scholar]
- Katzir I, Shani J, Wolf W, Chatterjee-Parti S, Berman E. Enhancement of 5-fluorouracil anabolism by methotrexate and trimetrexate in two rat solid tumor models, Walker 256 carcinosarcoma and Novikoff hepatoma, as evaluated by 19F-magnetic resonance spectroscopy. Cancer Invest. 2000;18:20–27. doi: 10.3109/07357900009023058. [DOI] [PubMed] [Google Scholar]
- King R, Bonfiglio R, Fernandez-Metzler C, Miller-Stein C, Olah T. Mechanistic investigation of ionization suppression in electrospray ionization. J Am Soc Mass Spectrom. 2000;11:942–950. doi: 10.1016/S1044-0305(00)00163-X. [DOI] [PubMed] [Google Scholar]
- Kong AN, Jusko WJ. Definitions and applications of mean transit and residence times in reference to the two-compartment mammillary plasma clearance model. J Pharm Sci. 1988;77:157–165. doi: 10.1002/jps.2600770213. [DOI] [PubMed] [Google Scholar]
- Kosovec JE, Egorin MJ, Gjurich S, Beumer JH. Quantitation of 5-fluorouracil (5-FU) in human plasma by liquid chromatography/electrospray ionization tandem mass spectrometry. Rapid Commun Mass Spectrom. 2008;22:224–230. doi: 10.1002/rcm.3362. [DOI] [PubMed] [Google Scholar]
- Kuan HY, Smith DE, Ensminger WD, Knol JA, DeRemer SJ, Yang Z, Stetson PL. Regional pharmacokinetics of 5-fluorouracil in dogs: role of the liver, gastrointestinal tract, and lungs. Cancer Res. 1998;58:1688–1694. [PubMed] [Google Scholar]
- Li KM, Rivory LP, Clarke SJ. Rapid quantitation of plasma 2′-deoxyuridine by high-performance liquid chromatography/atmospheric pressure chemical ionization mass spectrometry and its application to pharmacodynamic studies in cancer patients. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;820:121–130. doi: 10.1016/j.jchromb.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Licea-Perez H, Wang S, Bowen C. Development of a sensitive and selective LC-MS/MS method for the determination of alpha-fluoro-beta-alanine, 5-fluorouracil and capecitabine in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:1040–1046. doi: 10.1016/j.jchromb.2009.02.025. [DOI] [PubMed] [Google Scholar]
- Liu HY, Ding L, Yu Y, Chu Y, Zhu H. Comparison of three derivatization reagents for the simultaneous determination of highly hydrophilic pyrimidine antitumor agents in human plasma by LC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2012;893–894:49–56. doi: 10.1016/j.jchromb.2012.02.033. [DOI] [PubMed] [Google Scholar]
- Liu K, Zhong D, Zou H, Chen X. Determination of tegafur, 5-fluorouracil, gimeracil and oxonic acid in human plasma using liquid chromatography–tandem mass spectrometry. J Pharm Biomed Anal. 2010;52:550–556. doi: 10.1016/j.jpba.2010.01.026. [DOI] [PubMed] [Google Scholar]
- Lobo ED, Balthasar JP. Pharmacokinetic–pharmacodynamic modeling of methotrexate-induced toxicity in mice. J Pharm Sci. 2003;92:1654–1664. doi: 10.1002/jps.10431. [DOI] [PubMed] [Google Scholar]
- Loos WJ, de Bruijn P, van Zuylen L, Verweij J, Nooter K, Stoter G, et al. Determination of 5-fluorouracil in microvolumes of human plasma by solvent extraction and high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl. 1999;735:293–297. doi: 10.1016/s0378-4347(99)00414-4. [DOI] [PubMed] [Google Scholar]
- Miglioli PA, Businaro V, Manoni F, Berti T. Tissue distribution of methotrexate in rats. A comparison between intravenous injection as bolus or drip infusion. Drugs under experimental and clinical research. 1985;11:275–279. [PubMed] [Google Scholar]
- Moore MJ, Erlichman C, Thiessen JJ, Bunting PS, Hardy R, Kerr I, Soldin S. Variability in the pharmacokinetics of cyclophosphamide, methotrexate and 5-fluorouracil in women receiving adjuvant treatment for breast cancer. Cancer Chemother Pharmacol. 1994;33:472–476. doi: 10.1007/BF00686503. [DOI] [PubMed] [Google Scholar]
- Olsen EA. The pharmacology of methotrexate. J Am Acad Dermatol. 1991;25:306–318. doi: 10.1016/0190-9622(91)70199-c. [DOI] [PubMed] [Google Scholar]
- Peer CJ, McManus TJ, Hurwitz HI, Petros WP. Development and utilization of a combined LC-UV and LC-MS/MS method for the simultaneous analysis of tegafur and 5-fluorouracil in human plasma to support a phase I clinical study of oral UFT®/leucovorin. J Chromatogr B Analyt Technol Biomed Life Sci. 2012;898:32–7. doi: 10.1016/j.jchromb.2012.04.010. [DOI] [PubMed] [Google Scholar]
- Peters GJ, Lankelma J, Kok RM, Noordhuis P, van Groeningen CJ, van der Wilt CL, et al. Prolonged retention of high concentrations of 5-fluorouracil in human and murine tumors as compared with plasma. Cancer Chemother Pharmacol. 1993;31:269–276. doi: 10.1007/BF00685670. [DOI] [PubMed] [Google Scholar]
- Pinedo HM, Peters GF. Fluorouracil: biochemistry and pharmacology. J Clin Oncol. 1988;6:1653–1664. doi: 10.1200/JCO.1988.6.10.1653. [DOI] [PubMed] [Google Scholar]
- Pisano R, Breda M, Grassi S, James CA. Hydrophilic interaction liquid chromatography–APCI–mass spectrometry determination of 5-fluorouracil in plasma and tissues. J Pharm Biomed Anal. 2005;38:738–45. doi: 10.1016/j.jpba.2005.01.039. [DOI] [PubMed] [Google Scholar]
- Remaud G, Boisdron-Celle M, Morel A, Gamelin A. Sensitive MS/MS-liquid chromatography assay for simultaneous determination of tegafur, 5-fluorouracil and 5-fluorodihydrouracil in plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;824:153–160. doi: 10.1016/j.jchromb.2005.07.023. [DOI] [PubMed] [Google Scholar]
- Sabatini L, Barbieri A, Tosi M, Violante FS. A new high-performance liquid chromatographic/electrospray ionization tandem mass spectrometric method for the simultaneous determination of cyclophosphamide, methotrexate and 5-fluorouracil as markers of surface contamination for occupational exposure monitoring. J Mass Spectrom. 2005;40:669–674. doi: 10.1002/jms.840. [DOI] [PubMed] [Google Scholar]
- Sartori T, Murakami Seigi F, Cruz Pinheiro A, de Campos Machado A. Development and validation of a fast RP-HPLC method for determination of methotrexate entrapment efficiency in polymeric nanocapsules. J Chromatogr Sci. 2008;46:505–509. doi: 10.1093/chromsci/46.6.505. [DOI] [PubMed] [Google Scholar]
- Shah VP, Midha KK, Dighe S, McGilveray IJ, Skelly JP, Yacobi A, Layloff T, Viswanathan CT, Cook CE, McDowall RD. Analytical methods validation: bioavailability, bioequivalence and pharmacokinetic studies. Conference report. Eur J Drug Metab Pharmacokinet. 1991;16:249–255. doi: 10.1007/BF03189968. [DOI] [PubMed] [Google Scholar]
- Sparreboom A, Loos WJ, Nooter K, Stoter G, Verweij J. Liquid chromatographic analysis and preliminary pharmacokinetics of methotrexate in cancer patients co-treated with docetaxel. J Chromatogr B Biomed Sci Appl. 1999;735:111–119. doi: 10.1016/s0378-4347(99)00387-4. [DOI] [PubMed] [Google Scholar]
- van Kuilenburg AB, van Lenthe H, Maring JG, van Gennip AH. Determination of 5-fluorouracil in plasma with HPLC–tandem mass spectrometry. Nucleosides Nucleotides Nucleic Acids. 2006;25:1257–1260. doi: 10.1080/15257770600894741. [DOI] [PubMed] [Google Scholar]
- Wang K, Nano M, Mulligan T, Bush ED, Edom RW. Derivatization of 5-fluorouracil with 4-bromomethyl-7-methoxycoumarin for determination by liquid chromatography–mass spectrometry. J Am Soc Mass Spectrom. 1998;9:970–976. doi: 10.1016/S1044-0305(98)00067-1. [DOI] [PubMed] [Google Scholar]
- Wang Z, Zhou Q, Kruh GD, Gallo JM. Dose-dependent disposition of methotrexate in Abcc2 and Abcc3 gene knockout murine models. Drug Metab Dispos. 2011;39:2155–2161. doi: 10.1124/dmd.111.041228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi H, Cho HJ, Cho SM, Lee DG, Abd El-Aty A, Yoon SJ, Bae GW, Nho K, Kim B, Lee CH, Kim JS, Bartlett MG, Shin HC. Pharmacokinetic properties and antitumor efficacy of the 5-fluorouracil loaded PEG-hydrogel. BioMed Central Cancer. 2010;10:211. doi: 10.1186/1471-2407-10-211. [DOI] [PMC free article] [PubMed] [Google Scholar]