Abstract
It has been hypothesized that chronic psychological stress is associated with shorter telomere length; however, the mechanisms that link stress and telomere length are not well understood. To examine the interplay between biochemical factors related to stress arousal and cellular aging, we investigate the association between anabolic/catabolic (A/C) imbalance and leukocyte telomere length (LTL) in the Social Environment and Biomarkers of Aging Study (SEBAS) in Taiwan (N=925). SEBAS participants age 54 and older (mean age 68.3) with values for two anabolic hormones (serum dehydroepiandrosterone sulfate [DHEAS] and insulin growth factor [IGF]-1), four catabolic hormones (cortisol, epinephrine, norepinephrine, and interleukin-6 [IL-6]), and leukocyte telomere length were examined. We found that high IL-6 was associated with short LTL (≤0.88 T/S ratio; odds ratio [OR] 1.41, 95% confidence interval [CI] 1.04–1.92). Neither DHEAS/cortisol nor IGF-1/cortisol ratio was associated with telomere length; however, a high A/C imbalance summary score was associated with a greater odds of having a short LTL relative to long LTL (OR 1.19, 95% CI 1.05–1.35). These results indicate that our A/C imbalance score, defined by several anabolic and catabolic biochemical factors, may be one mechanism through which psychological stress is associated with short leukocyte telomere length and possibly cellular senescence.
INTRODUCTION
Psychoneuroendocrinology, or the study of how the mind, brain, and hormonal functions interact, provides a glimpse into the neuroanatomy of the endocrine system and has contributed to our understanding of the connections between brain and peripheral processes. Hormonal levels are known to coordinate adaptive responses to maintain homeostasis and regulate peripheral physiologic processes (Sapolsky, Krey, & McEwen, 1986), and these responses may in-turn result in adverse effects on health over time. Changes in hormone levels are thought to play an important role in physiological aging processes, which have long been associated with altered hormonal levels. It has been proposed, however, that the effects of aging on hormone levels (e.g., declines in insulin-like growth factor [IGF]-1 and growth hormone [GH], and increases in cortisol) are not a definitive part of the aging process and may be driven, in part, by chronic stress rather than aging per se (Epel, Burke, & Wolkowitz, 2007).
Chronic stress is thought to result in a shift of hormone balance: to low anabolic hormone levels and high catabolic hormone levels. Low levels of anabolic hormones elicit: increased skeletal and lean mass, prevention of adiposity, and the promotion of elevated cortisol levels (considered a catabolic factor) (Epel, 2009). Given this, both anabolic and catabolic hormones are thought to play critical roles in aging and processes related to physiological stress.
Telomeres, which are protein-DNA structures that protect the ends of chromosomes from fusion and degradation, have been considered a marker of cellular aging (Blackburn, 2010). The accelerated shortening of telomere length in peripheral blood cells has been reported in a number of conditions and diseases, including cardiovascular disease, rheumatoid arthritis, and HIV (Effros et al., 2005) and has been associated with longevity (Cawthon et al., 2003; Monaghan, 2012). A growing body of studies have documented the relationship between chronic psychological stress and telomere length and telomerase activity (Shalev et al., 2013a), particularly among caregivers of patients with Alzheimer’s Disease (Damjanovic et al., 2007), mothers with chronically ill children (Epel et al., 2004), and children experiencing adverse life events (Shalev et al., 2013b).
The mechanisms that link psychological stress and telomere length, however, are not well understood. One hypothesis is that chronic stress shifts the balance of hormones to low levels of anabolic hormones (e.g., dehydroepiandrosterone [DHEA], growth hormone [GH], and insulin-like growth factor [IGF]-1) and high levels of catabolic hormones (e.g., cortisol, adrenaline [epinephrine], and cytokines). This shift in hormonal balance, termed anabolic/catabolic (A/C) imbalance (e.g., high cortisol and low growth hormones and androgens), is thought to be linked to systemic inflammation (Davis et al., 2008) and oxidative stress (Yamaji et al., 2009) which consequently may promote leukocyte cellular aging (Demissie et al., 2006; Dowd et al., 2013).
There has been some work linking catabolic hormones to telomere length. Cortisol, a hormone involved in the hypothalamic-pituitary-adrenocortical (HPA) axis, has been implicated as one of the biological mechanisms that links environmental stress exposures to telomere length. For example, in vitro exposure of human T lymphocytes (white blood cells that mature in the thymus) to cortisol resulted in a reduction in telomerase activity (Choi, Fauce, Effros, 2008). The decreased activity of telomerase, a cellular enzyme that stabilizes telomere length through its reverse transcriptase activity, promotes telomere shortening and cell senescence. Additionally, elevated levels of epinephrine were associated with low leukocyte telomerase activity in a sample of healthy women (Epel et al., 2006).
Several previous studies have also found positive associations between telomere length and IGF-1, though relationships with GH and DHEAS appear not to have been assessed previously (Aulinas et al, 2013). Anabolic hormones may play an important role in cellular aging. For instance, low levels of DHEA and IGF-1 have been associated with a greater risk of mortality among men (Maggio et al., 2007), and GH and IGF-1 have been linked to shorter lifespan in several animal models (Berryman et al., 2008). The importance of considering anabolic hormones in the relationship between psychological stress and telomere length is further evidenced by the crucial balance between anabolic and catabolic hormones. This is illustrated through the ability of DHEA to block cortisol-related neurotoxicity (Kimonides et al., 1999), which is a consequence of neuronal damage due to chronic elevations in cortisol (Behl et al., 1997; McIntosh & Sapolsky, 1996).
To further our understanding of the interplay between biochemical factors related to stress arousal and cellular aging, we investigate the links between telomere length and: individual A/C hormones, two A/C ratios, and an A/C imbalance summary score in a population-based sample of older Taiwanese adults. We hypothesize that a higher A/C ratio and higher A/c imbalance summary score, indicating abnormal levels of more A/C hormones, will be associated with short telomere length.
METHODS
The current analysis is based on the Social Environment and Biomarkers of Aging Study (SEBAS). SEBAS comprises a random subsample of respondents from the Taiwan Longitudinal Study of Aging (TLSA), a survey of older Taiwanese adults that began in 1989. Among individuals age 54 and older selected for SEBAS, 1497 (92%) were interviewed in 2000. Weeks after the interview, SEBAS respondents who participated in the medical exam (n=1023; 68% of those interviewed) were asked to fast overnight, to collect a 12 hour overnight urine sample (7pm to 7am for collection of cortisol and catecholamines), and to report to a nearby hospital the next morning. As part of the hospital visit, anthropometric measurements, blood pressure readings, and blood samples were taken. Nearly all participants provided testable blood and urine samples, which were analyzed at Union Clinical Laboratories (UCL) in Taipei.
DNA was extracted from whole blood by UCL using trimethyl ammonium bromide salts (DTAB and CTAB). Samples were diluted based on Spectrophotometer measurements 0.5 and 10 ng/ul used in quantitative polymerase chain reaction (qPCR). This suggested that 5 ng of the control gene DNA per sample were expected. Leukocyte telomere length (LTL) was then measured at the University of Washington using a qPCR-based technique similar to that of Cawthon, 2002 and Risques et al., 2007. Two PCRS were performed for each sample to: (1) amplify the telomeric DNA, and (2) amplify the single-copy control gene (36B4, acidic ribosomal phosphoportein PO). Comparing the telomere repeat sequence copy number to 36B4 served as an internal control to normalize the initial amount of DNA. We used a four-point standard curve, based on two-fold serial DNA dilutions of 10 to 1.25 ng, to run PCRs, which enabled transformation of cycle threshold into ng of DNA. LTLs were measured in triplicates and median lengths were used for statistical analysis. LTLs that were outside of the acceptable range for 36B4 (< 1 ng or 36b4 >6 ng; n=33) were excluded from the current analysis. LTL was calculated as the ratio of telomere/single-copy control gene (T/S ratio), which is proportional to the average telomere length in a cell (Cawthon, 2002). Blinded reproducibility experiments were periodically conducted to ensure accurate measurement, and two control samples per experiment were performed in each experiment to enable normalization between experiments. The coefficient of variation (CV) or inter-assay variability for qPCR was 7%. This assay has been previously used (Aviv et al., 2011), with an excellent correlation with the DNA blot (or Southern blot) method and with an inter-assay CV of 6.5%, which is similar to the CV of the current study. Given our interest in cellular aging and the detriments of shortened telomeres, we examine short LTL as determined by being in the lowest sample-based quartile (≤0.88 T/S ratio).
Using data from the SEBAS samples collected in 2000, we examined two anabolic hormones (serum DHEAS [DHEA in its sulfate form] and IGF-1) and four catabolic hormones (cortisol, epinephrine, norepinephrine, and cytokine interleukin-6 [IL-6]). DHEAS was used because it remains in the bloodstream longer than DHEA and because it exhibits less diurnal variation (Kroboth et al., 1999). DHEAS, IGF-1, and IL-6 levels were measured using enzyme immunoassay (EIA) from Diagnostic Products Corporation; Nichols Institute Diagnostics; and Endogen, Pierce Biotechnology (respectively). Urinary cortisol was measured using high-pressure liquid chromatography (HPLC), ultraviolet detection (UV). Urinary epinephrine and norepinephrine were measured using HPLC, electrochemical detection (ECD) (Chromosystems). The CV for IGF-1 and IL-6 were slightly above the 10% optimal cutoff (13.6% and 12.6%, respectively); however, the CVs for cortisol, epinephrine, and norepinephrine were closer to or below the optimal cutoff (10.8%, 8.9%, and 4.9%, respectively).
At-risk levels of DHEAS and IGF-1 were determined based on cutpoints from the lowest quartile of the study sample; at-risk levels of epinephrine, norepinephrine, and IL-6 were based on the highest quartile of the study sample. Since both high and low cortisol has reported effects through alternative pathways on cell aging, we investigate two separate at-risk cutpoints based on the highest and lowest quartile of cortisol. Individuals with telomere length in the bottom half of the sample study were considered as having short telomere length.
A/C imbalance was considered using three parameterizations. First, individual associations of at-risk levels of each anabolic and catabolic hormone to telomere length were investigated. Second, we also considered the balance between anabolic and catabolic hormones by examining DHEAS/cortisol and IGF-1/cortisol ratios (as often used in the literature, e.g., Cruess et al., 1999; Daly, Rich, Kelin, 1998). Third, a summary measure of A/C imbalance was created for each respondent by summing the number of at-risk anabolic and catabolic markers (range 0–6; individuals with high or low cortisol levels received one point for the score). Logistic regression models were used to determine the relationship between at-risk levels of individual anabolic and catabolic hormones, DHEAS/cortisol and IGF-1/cortisol ratios, and the summary measure of A/C imbalance with short telomere length. All models were adjusted for age, sex, marital status, and urban/rural residency using STATA Version 11 (StataCorp, 2007).
RESULTS
Table 1 reports the sample characteristics. On average, participants were 68 years old (range 54–91) with a greater proportion of men (57%). Slightly more respondents resided in an urban area compared to a rural area (54%). Mean levels of each anabolic and catabolic hormone were within the range of other population-based surveys (Crimmins et al., 2008), and mean LTL was 0.9 (standard deviation [SD] 0.02), which is similar to that of prior studies that report LTL in T/S ratios (e.g., Zhang et al, 2014). Compared to participants with long LTL (>0.88), we find that the short LTL (≤ 0.88) group was comprised of participants who were younger, with fewer men, fewer urban residents and more married individuals. Moreover, participants with short LTL had lower DHEAS, epinephrine, IL-6, and lower scores for all of the three ratios (DHEAS/cortisol, IGF-1/cortisol, and A/C imbalance ratio), relative to participants with LTL. We considered the correlations between LTL and the demographic measures considered, and we observed a significant association between LTL and age (r=−0.20, p<.001), and LTL and sex (r=−011, p<.01).
Table 1.
Sample characteristics of Taiwanese older adults
| At-risk cutoff | N | Mean ± SD or %
|
|||
|---|---|---|---|---|---|
| All | Stratified by LTL
|
||||
| Long (>0.88) | Short (≤ 0.88) | ||||
| Age | 943 | 68.3 ± 8.5 | 70.1 ± 8.3 | 66.5 ± 8.3 | |
| Men (%) | 943 | 57 | 63 | 51 | |
| Urban (vs. rural) residency (%) | 943 | 54 | 56 | 52 | |
| Married (vs. non-married) (%) | 943 | 71 | 68 | 73 | |
| Anabolic (A) | |||||
| DHEAS (ug/dl) | ≤ 39.9 | 942 | 81.2 ± 59.7 | 81.9 ± 60.8 | 80.5 ± 58.7 |
| IGF-1 (pg/ml) | ≤ 68.7 | 942 | 104.8 ± 48.6 | 101.9 ± 44.8 | 107.7 ± 51.9 |
| Catabolic (C) | |||||
| Cortisol (low) (u/g creatinine) | ≤ 12.53406 | 939 | 28.5 ± 53.4 | 24.4 ± 24.8 | 32.5 ± 71.3 |
| Cortisol (high) (u/g creatinine) | ≥ 29.97763 | ||||
| Epinephrine (u/g creatinine) | ≥3.671971 | 939 | 2.6 ± 2.6 | 2.7 ± 2.6 | 2.6 ± 2.6 |
| Norepinephrine (u/g creatinine) | ≥27.09205 | 939 | 22.0 ± 9.9 | 21.9 ± 9.8 | 22.1 ± 10.0 |
| Interleukin-6 (pg/ml) | ≥ 3.9115 | 929 | 3.6 ± 5.7 | 3.8 ± 4.8 | 3.4 ± 2.8 |
| A/C Imbalance | |||||
| DHEAS/cortisol ratio | 938 | 5.1 ± 5.8 | 5.3 ± 5.6 | 4.9 ± 5.9 | |
| IGF-1/cortisol ratio | 938 | 6.9 ± 7.2 | 7.0 ± 7.2 | 6.8 ± 7.1 | |
| A/C imbalance summary measure | 925 | 2.2 ± 1.1 | 2.4 ± 1.1 | 2.1 ± 1.1 | |
| Telomere length (T/S ratio) | ≤ 0.88 | 943 | 0.9 ± 0.2 | ||
LTL = leukocyte telomere length
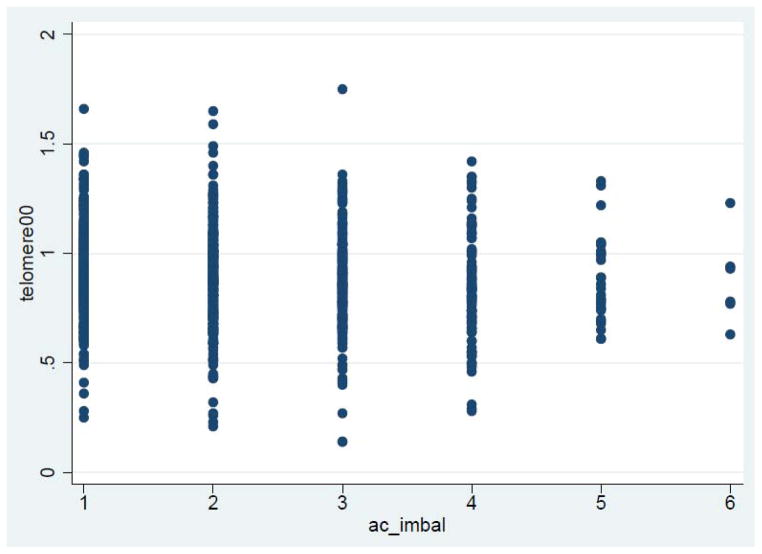
Table 2 reports the odds ratios (OR) for predicting short telomere length from at-risk levels of individual anabolic and catabolic measures, from the two A/C ratios, and the A/C imbalance summary measure. After controlling for age, sex, and urban/rural residency, we found that high IL-6 was the only individual biochemical factor that was associated with short telomere length, though this relationship disappears if LTL is treated as a continuous variable (p=0.82). Neither of the two A/C ratios (DHEAS/cortisol nor IGF-1/cortisol) was associated with short telomere length; however, a higher A/C imbalance summary score was associated with a greater odds of having a short telomere length (odds ratio 1.19; 95% confidence interval 1.05–1.35), a result that is reproducible using LTL as a continuous variable (beta=−0.02, p=0.02). The scatterplot of telomere length and A/C imbalance summary score, illustrated in Figure 1, suggests the absence of a dose-response relationship. No significant interactions between sex and any of the biochemical factors or A/C ratio measures were observed.
Table 2.
Odds ratios from logistic regression models predicting short telomere length by at-risk levels of biochemical factors, A/C imbalance ratios, and the A/C imbalance summary measure
| Biochemical Factor | N | Short Telomere Length
|
|---|---|---|
| OR (95% CI) | ||
| Anabolic (A) | ||
| DHEAS (≤ 39.9 ug/dL) | 942 | 1.27 (0.93–1.75) |
| IGF-1 (≤ 68.7 pg/mL) | 942 | 1.08 (0.79–1.47) |
| Catabolic (C) | ||
| Cortisol (≤ 12.53406 u/g creatinine) | 939 | 1.23 (0.91–1.68) |
| Cortisol (≥ 29.97763 u/g creatinine) | 939 | 0.83 (0.61–1.13) |
| Epinephrine (≥3.671971 u/g creatinine) | 939 | 1.24 (0.92–1.70) |
| Norepinephrine (≥27.09205 u/g creatinine) | 939 | 1.19 (0.87–1.62) |
| Interleukin-6 (≥ 3.9115pg/ml) | 929 | 1.41 (1.04–1.92)† |
| A/C Imbalance | ||
| DHEAS/Cortisol ratio | 938 | 1.01 (0.99–1.04) |
| IGF-1/Cortisol ratio | 938 | 1.01 (0.99–1.03) |
| A/C imbalance summary measure | 925 | 1.19 (1.05–1.35)† |
OR=odds ratio; CI=confidence interval; DHEAS=Dehydroepiandrosterone sulfate; IGF-1=insulin-like growth factor-1
Adjusts for age, sex, marital status, and urban/rural residence
p<.05
Figure 1.
Scatterplot of A/C imbalance summary measure by telomere length (continuous measure)
A/C = anabolic/catabolic
DISCUSSION
Our findings suggest that cytokine IL-6 and a summary measure of A/C imbalance are associated with shortened telomere length. The relationship between elevated circulating IL-6 levels and telomere attrition has been previously reported (Shiels et al., 2011), but our reported link between the A/C imbalance summary measure and telomere length is a new contribution to the literature. This result suggests that A/C imbalance, as defined by multiple at-risk levels of anabolic and catabolic biochemical factors, may be one mechanism through which psychological or environmental stress is associated LTL and possibly cellular senescence.
Notably, short telomere length was not significantly associated with levels of individual anabolic and catabolic hormones, nor with ratios of DHEAS or IGF-1 to cortisol. However, the effect sizes are large and mostly positive, and the confidence intervals are wide despite a relatively large sample size. Only the effect size for high cortisol goes in the opposite direction of predictions, implying a potential monotonic negative association between cortisol level and risk of shortened telomere length. This finding for cortisol likely explains why the anabolic/cortisol ratios were also not significantly associated with risk of short telomere length.
One possible explanation for both the wide confidence intervals and for the better performance of the A/C imbalance summary measure compared to the other measures is that there may be multiple potential pathways linking psychological stress to short telomere length. This would result in substantially weakened signals for each marker alone, but perhaps a stronger signal for appropriate composite measures. The A/C imbalance score we used here is one possibility, but it is possible there are other scores that are still more informative, such as a statistical distance among markers (Cohen et al., 2013). Which summary measures perform best may indeed be informative about the nature of the underlying processes.
Although most of the relationships we tested for were not significant, we can still ask if they broadly agree with or contradict other findings in the literature. As noted above, the telomere-IL-6 relationship has been previously described (Shiels et al., 2011) and is thus concordant with previous studies. IGF-1 and telomere length have been found to be positively and often strongly associated in a number of studies (Barbieri et al., 2009, Aulinas et al., 2013); while the effect size is consistent with this in terms of direction, it is surprising that we did not detect a stronger, significant effect, given our relatively large sample size. For the cortisol-telomere length relationship, previous studies are more varied (Aulinas et al., 2013), though most have shown a negative relationship between telomere length and cortisol levels, the opposite of the trend we detect. In the current study, the absence of an association between LTL and many of the hormones examined may suggest that either: (1) a relationship does exist, but we were unable to detect this in our population, or (2) a relationship does not exist, and our null findings reflect a ‘true’ absence of an association. If an association does in fact exist, our study may not have observed this given differences in age and racial composition. For instance, Barbieri et al. (2009) observe a positive relationship between LTL and circulating IGF-1 levels in 476 healthy, unrelated Caucasian men and women residing in the West Coast of Southern Italy, who were age 16–104 years after adjusting only for age. Although we are unable to definitively pinpoint which of the two scenarios mentioned above is represented in the correct study, we acknowledge the possibility that given our moderate sample size for detecting an effect of A/C hormones on LTL, it is possible that, as we observed, no such association between LTL and anabolic and catabolic hormone levels exists (with the exception of IL-6).
This study has several important limitations. The availability of both telomere length and hormones at a single time point hinders our ability to make causal inferences based on the detected associations. In particular, telomeres are thought to shorten progressively throughout life, which would imply that current telomere length may be a reflection of cumulative shortening over the lifespan; we might thus expect only weak correlations between current telomere length and current measures of the relevant pathways, even if these pathways play an important role in determining telomere length. The available hormones do not represent the full spectrum of anabolic and catabolic hormones, and we are thus not capable of perfectly measuring A/C imbalance. Moreover, due to unavailability of information on hormone replacement therapy (HRT) in women, we do not consider the role of HRT in the observed relationship between cortisol and short telomere length. Previous work indicates that estrogen deficiency inhibits telomerase maintenance of telomeres (Bayne et al., 2011; Pines, 2013), which may confound the relationships between anabolic and catabolic hormones and telomere length, particularly among women. Lastly, we report short telomere length in our current analysis, although alternate methods for considering short telomere length and ‘telomere shortening’ exist. In our sensitivity analyses (not shown; results available upon result), we considered telomere length as a continuous measure, and we found no significant associations between telomere length and the individual A/C markers nor the two A/C ratios: DHEAS/cortisol and IGF-1/cortisol. However, higher A/C imbalance summary scores were significantly associated with lower telomere length (beta=−0.02, p=0.02).
Much debate remains with respect to indexing of ‘telomere shortening.’ This includes whether consideration of cross-sectional data is sufficient or longitudinal studies are required, and how to determine the cutpoint for ‘short telomere length’ (e.g., quartile-based or median-based depending on the population of interest, or if a single cutpoint across all populations with varying racial and age distributions is appropriate). Future studies should address the concerns surrounding the measurement and determination of ‘telomere shortening,’ as additional population-based surveys with large sample sizes are incorporating LTL measurement at more than one timepoint. Additionally, future longitudinal studies would benefit from examining potential interactions between anabolic-catabolic hormones, telomerase activity, and telomere length in order to understand how the dynamics of stressors may affect telomere shortening over the life course. Additionally, stability of results across populations will be important to verify: are the effects detected here general biological processes, or are they specific to a Taiwanese context (and if so, why)? How might cultural or genetic factors modulate the effect of stress on telomere shortening, and thereby aging? Lastly, the literature on the mechanisms linking short telomere length with stress, particularly A/C hormones and telomere length, would benefit from consideration of indicators of physical and mental health.
In summary, in this population-based study of older Taiwanese adults, we found that high IL-6 levels were associated with short telomere length and a higher A/C imbalance summary measure was associated with a greater odds of having a short telomere length. Our findings underscore the role of anabolic and catabolic biochemical factors in health conditions associated with telomere length, including the literature linking short telomere length with depression. Given our knowledge that patients with major depression have abnormal hormone levels (Pariante & Lightman, 2008) or differ in genetic variation associated with HPA axis hyperactivity (Schatzberg et al., 2014) and previous findings on the association between major depressive disorder and short telomere length (Verhoeven et al., 2013; Zhang et al., 2014), knowledge of an individual’s A/C summary score may be beneficial in identifying patients at highest risk of developing depressive symptoms. Efforts to address anabolic and catabolic biochemical levels in clinical settings may contribute to our ability to detect the adverse health conditions associated with telomere length and potentially identify mechanisms to diminish and treat the consequences linked to these conditions.
References
- Aulinas A, Ramírez MJ, Barahona MJ, Mato E, Bell O, Surralles J, Webb SM. Telomeres and endocrine dysfunction of the adrenal and GH/IGF-1 axes. Clin Endocrinol. 2013;79:751–759. doi: 10.1111/cen.12310. [DOI] [PubMed] [Google Scholar]
- Aviv A, Hunt SC, Lin J, Cao X, Kimura M, Blackburn E. Impartial comparative analysis of measurement of leukocyte telomere length/DNA content by Southern blots and qPCR. Nucleic Acids Res. 2011;39:e134. doi: 10.1093/nar/gkr634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbieri M, Paolisso G, Kimura M, Gardner JP, Boccardi V, Papaa M, Hjelmborg JV, Christensen K, Brimacombe M, Nawrot TS, Staessen JA, Pollak MN, Aviv A. Higher circulating levels of IGF-1 are associated with longer leukocyte telomere length in healthy subjects. Mech Ageing Dev. 2009;130:771–776. doi: 10.1016/j.mad.2009.10.002. http://www.sciencedirect.com/science/article/pii/S004763740900147X-aff3 http://www.sciencedirect.com/science/article/pii/S004763740900147X-aff2. [DOI] [PubMed] [Google Scholar]
- Bayne S, Li H, Jones ME, Pinto AR, van Sinderen M, Drummond A, Simpson ER, Liu JP. Estrogen deficiency reversibly induces telomere shortening in mouse granulosa cells and ovarian aging in vivo. Protein Cell. 2011;2:333–346. doi: 10.1007/s13238-011-1033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behl C, Lezoualc’h F, Trapp T, Widmann M, Skutella T, Holsboer F. Glucocorticoids enhance oxidative stress-induced cell death in hippocampal neurons in vitro. Endocrinol. 1997;138:101–106. doi: 10.1210/endo.138.1.4835. [DOI] [PubMed] [Google Scholar]
- Berryman DE, Christiansen JS, Thorner MO, Kopchick JJ. Role of the GH/IGF-1 axis in lifespan and healthspan: Lessons from animal models. Growth Horm IGF Res. 2008;18:455–471. doi: 10.1016/j.ghir.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn EH. Telomeres and telomerase: The means to the end (Nobel Lecture) Angew Chem Int Ed. 2010;49:7405–7421. doi: 10.1002/anie.201002387. [DOI] [PubMed] [Google Scholar]
- Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30:e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawthon RM, Smith KR, O’Brien E, Sivatchenko AA, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003;361:393–395. doi: 10.1016/S0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
- Cohen AA, Milot E, Yong J, Seplaki CL, Fülöp T, Bandeen-Roche K, Fried LP. A novel statistical approach shows evidence for multi-system physiological dysregulation during aging. Mech Ageing Dev. 2013;34:110–117. doi: 10.1016/j.mad.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Fauce SR, Effros RB. Reduced telomerase activity in human T lymphocytes exposed to cortisol. Brain Behav Immun. 2008;22:600–605. doi: 10.1016/j.bbi.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimmins E, Vasunilashorn S, Kim JK, Alley D. Biomarkers related to aging in human populations. Adv Clin Chem. 2008;46:161–216. doi: 10.1016/s0065-2423(08)00405-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruess DG, Antoni MH, Kumar M, Ironson G, McCabe P, Fernandez JB, Fletcher M, Schneiderman N. Cognitive-behavioral stress management buffers decreases in dehydroepiandrosterone sulfate (DHEA-S) and increases in the cortisol/DHEA-S ratio and reduces mood disturbance and perceived stress among HIV-seropositive men. Psychoneuroendocrinology. 1999;24:537–549. doi: 10.1016/s0306-4530(99)00010-4. [DOI] [PubMed] [Google Scholar]
- Daly RM, Rich PA, Kelin R. Hormonal responses to physical training in high- level peripubertal male gymnasts. Eur J Appl Physiol. 1998;79:74–81. doi: 10.1007/s004210050476. [DOI] [PubMed] [Google Scholar]
- Damjanovic AK, Yang Y, Glaser R, Kiecolt-Glaser JK, Nguyen H, Laskowski B, Zou Y, Beversdorf DQ, Weng NP. Accelerated telomere erosion is associated with a declining immune function of acregivers of Alzheimer’s disease patients. J Immunol. 2007;179:4249–4254. doi: 10.4049/jimmunol.179.6.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MC, Zautra AJ, Younger J, Motivala SJ, Attrep J, Irwin MR. Chronic stress and regulation of cellular markers of inflammation in Rheumatoid Arthritis: Implications for fatigue. Brain Behav Immun. 2008;22:24–32. doi: 10.1016/j.bbi.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demissie S, Levy D, Benjamin EJ, Cupples LA, Gardner JP, Herbert A, Kiumra M, Larson MG, Meigs JB, Keaney JF, Aviv A. Insulin resistance, oxidative stress, hypertension, and leukocyte telomere length in men from the Framingham Heart Study. Aging Cell. 2006;5:325–330. doi: 10.1111/j.1474-9726.2006.00224.x. [DOI] [PubMed] [Google Scholar]
- Dowd JB, Bosch JA, Steptoe A, Blackburn EH, Lin J, Ree-Clayton E, Aiello AE. Cytomegalovirus is associated with reduced telomerase activity in the Whitehall II cohort. Exp Gerontol. 2013;48:385–390. doi: 10.1016/j.exger.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effros RB, Dagarag MD, Spaulding CC, Man J. The role of CD8+ T cell replicative senescence in human aging. Immunol Rev. 2005;205:147–157. doi: 10.1111/j.0105-2896.2005.00259.x. [DOI] [PubMed] [Google Scholar]
- Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci USA. 2004;101:17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES, Burke HM, Wolkowitz OM. Psychoneuroendocrinology of Aging: Focus on anabolic and catabolic hormones. In: Aldwin C, Spiro A, Park C, editors. Handbook of Health Psychology of Aging. Guildford Press; 2007. pp. 119–141. [Google Scholar]
- Epel ES, Lin J, Wilhelm FH, Wolkowitz OM, Cawthon R, Adler NE, Dolbier C, Mendes WB, Blackburn EH. Cell aging in relation to stress arousal and cardiovascular disease risk factors. Psychoneuroendocrinology. 2006;31:277–287. doi: 10.1016/j.psyneuen.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Kimonides VG, Spillantini MG, Sofroniew MV, Fawcett JW, Herbert J. Dehydroepiandrosterone antagonizes the neurotoxic effects of corticosterone and translocation of stress-activated protein kinase 3 in hippocampal primary cultures. Neuroscience. 1999;89:429–436. doi: 10.1016/s0306-4522(98)00347-9. [DOI] [PubMed] [Google Scholar]
- Kroboth PD, Salek FS, Pittenger AL, Fabian TJ, Frye RF. DHEA and DHEA-S: a review. J Clin Pharmacol. 1999;39:327–348. doi: 10.1177/00912709922007903. [DOI] [PubMed] [Google Scholar]
- Maggio M, Lauretani F, Ceda GP, Bandinelli S, Ling SM, Metter EJ, Artoni A, Carassale L, Cazzato A, Ceresini G, Guralnik JM, Basaria S, Valenti G, Ferrucci L. Relationship between low levels of anabolic hormones and 6-year mortality in older men: the aging in the Chianti Area (InCHIANTI) study. Arch Intern Med. 2007;167:2249–2254. doi: 10.1001/archinte.167.20.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcondes AM, Bair S, Rabinovitch PS, Gooley T, Deeg HJ, Risques R. No telomere shortening in marros stroma from patients with MDS. Ann Hematol. 2009;88:623–628. doi: 10.1007/s00277-008-0649-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh LJ, Sapolsky RM. Glucocorticoids increase the accumulation of reactive oxygen species and enhance Adriamycin-induced toxicity in neuronal culture. Exp Neurol. 1996;141:201–206. doi: 10.1006/exnr.1996.0154. [DOI] [PubMed] [Google Scholar]
- Monaghan P. Telomeres and longevity. Aging. 2012;4:76–77. doi: 10.18632/aging.100437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pariante CM, Lightman SL. The HPA axis in major depression: classical theories and new developments. Trends in Neurosci. 2008;31:464–468. doi: 10.1016/j.tins.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Pines A. Telomere length and telomerase activity in the context of menopause. Climacteric. 2013;16:629–631. doi: 10.3109/13697137.2013.812603. [DOI] [PubMed] [Google Scholar]
- Risques RA, Vaughan TL, Li S, Odze RD, Blount PL, Ayub K, Gallaher JL, Reid BJ, Rabinovitch PS. Leukocyte telomere length predicts cancer risk in barrett’s esophagus. Cancer Epidemiol Biomarkers Prev. 2007;16:2679–2655. doi: 10.1158/1055-9965.EPI-07-0624. [DOI] [PubMed] [Google Scholar]
- Sapolsky R, Krey L, McEwen B. The neuroendocrinology of stress and aging: The glucocorticoid cascade hypothesis. Endocr Rev. 1986;7:284–301. doi: 10.1210/edrv-7-3-284. [DOI] [PubMed] [Google Scholar]
- Schatzberg AF, Keller J, Tennakoon L, Lembke A, Williams G, Sarginson JE, Lazzeroni LC, Murphy GM. HPA axis genetic variation, cortisol and psychosis in major depression. Mol Psychiatry. 2014;19:220–227. doi: 10.1038/mp.2013.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev I, Entringer S, Wadhwa PD, Wolkowitz OM, Puterman E, Lin J, Epel ES. Stress and telomere biology: a lifespan perspective. Psychoneuroendocrinology. 2013a;38:1835–1842. doi: 10.1016/j.psyneuen.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev I, Moffitt TE, Sugden K, Williams B, Houts RM, Danese A, Mill J, Arseneault L, Caspi A. Exposure to violence during childhood is associated with telomere erosion from 5 to 10 years of age: a longitudinal study. Mol Psychiatry. 2013b;18:576–581. doi: 10.1038/mp.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiels PG, McGlynn LM, MacIntyre A, Johnson PCD, Batty GD, Burns H, Cavanagh J, Deans KA, Ford I, McConnachie A, McGinty A, McLean JS, Millar K, Sattar N, Tannahill C, Velupillai YN, Packard CJ. Accelerated telomere attrition is associated with relative household income, diet and inflammation in the pSoBid Cohort. PLoS One. 2011;6:e22521. doi: 10.1371/journal.pone.0022521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoeven JE, Révész D, Epel ES, Lin J, Wolkowitz OM, Penninx BWJH. Major depressive disorder and accelerated celular aging: results from a large psychiatric cohort study. Mol Psychiatry. 2013 doi: 10.1038/mp.2013.151. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Yamaji M, Tsutamoto T, Kawahara C, Nishiyama K, Yamamoto T, Fujii M, Horie M. Serum cortisol as a useful predictor of cardiac events in patients with chronic heart failure. Impact of Oxidative Stress. Circ Heart Fail. 2009;2:608–615. doi: 10.1161/CIRCHEARTFAILURE.109.868513. [DOI] [PubMed] [Google Scholar]
- Zhang L, Hu XZ, Li X, Li H, Smerin S, Russell D, Ursano RJ. Telomere length – A cellular aging marker for depression and post-traumatic stress disorder. Med Hypotheses. 2014 doi: 10.1016/j.mehy.2014.04.033. epub ahead of print. [DOI] [PubMed] [Google Scholar]