Abstract
Objective
To estimate gestational-age-specific risks of fetal death in pregnancies complicated by preeclampsia.
Methods
Population-based cohort study comprising all singleton births (N=554,333) without preexisting chronic hypertension recorded in the Norwegian Medical Birth Registry from 1999-2008. Additional data come from a subset of preeclamptic pregnancies enrolled in the Norwegian Mother and Child Cohort Study with available medical records (N=3037). The risk of fetal death, expressed per 1,000 fetuses exposed to preeclampsia, was calculated using a life-table approach.
Results
Preeclampsia was recorded in 3.8% (n=21,020) of all pregnancies. Risk of stillbirth was 3.6/1000 overall and 5.2/1000 among pregnancies with preeclampsia (relative risk (RR) =1.45, 95% confidence interval (CI) =1.20 to 1.76). However, relative risk of stillbirth was markedly elevated with preeclampsia in early pregnancy. In week 26 there were 11.6 stillbirths per 1000 pregnancies with preeclampsia, compared with 0.1 stillbirth per 1000 pregnancies without, relative risk 86 (95% CI=46 to 142). Fetal risk with preeclampsia declined as pregnancy advanced, but at 34 weeks remained more than sevenfold higher than pregnancies without preeclampsia.
Conclusion
For clinical purposes, the fetal risk of death associated with preeclampsia begins when preeclampsia becomes clinically apparent. Using a method that takes into account the clinical diagnosis of preeclampsia and the population of fetuses at risk, we find a remarkably high relative risk of fetal death among pregnancies diagnosed with preeclampsia in the preterm period.
INTRODUCTION
Preeclampsia, a pregnancy-related condition characterized by hypertension and proteinuria, is associated with increased fetal death.(1,2) Preeclampsia arising in the preterm period is of particular concern because it is generally considered to be more dangerous to both the mother and fetus.(3) Paradoxically, efforts to quantify the risk of stillbirth at each gestational week often suggest that the risk with preeclampsia (compared with normotensive pregnancies) is greater at term than at preterm.(1,4-6)
While the pathological origins of preeclampsia likely occur during placentation, the clinical signs and symptoms typically do not emerge until after 20 weeks gestation.(7) The most relevant estimate of fetal risk in the presence of preterm preeclampsia would be one that considers the timing of preeclampsia diagnosis – a diagnosis that often occurs well before the time of delivery. Detailed clinical records to determine the week in which preeclampsia is diagnosed are seldom available for the large study populations required to estimate fetal mortality. We used data from the Medical Birth Registry of Norway, supplemented by detailed antenatal records from a subset of those births, to estimate gestational-week-specific fetal mortality in the presence of preeclampsia.
MATERIALS AND METHODS
The Medical Birth Registry of Norway records all live births and fetal deaths after 12 weeks of gestation.(8) We selected for analysis all singletons born from 1999 through 2008 to mothers with no registered diagnosis of pre-existing hypertension (n = 564,753). We restricted analysis to pregnancies lasting at least 24 completed weeks but no longer than 42 weeks based on routine early ultrasound for 98% of all deliveries(9) and last menstrual period for the remainder. To avoid large errors in gestational age, we excluded infants with gestational-age-specific birth weights more than 5 standard deviations above the mean.(10) These several criteria excluded 2% of births, leaving 554,333 pregnancies for analysis. Review of the antenatal charts was carried out in accordance with the Medical Birth Registry regulation(11) and received appropriate ethical review and approval from the Medical Birth Registry of Norway and the University of North Carolina. The Medical Birth Registry of Norway approved the use of de-identified data for this analysis.
In Norway, pregnant women carry an antenatal card to each prenatal visit, where a midwife or physician records blood pressure and proteinuria. A separate study was conducted within the Medical Birth Registry to validate the registration of preeclampsia for preeclamptic pregnancies recorded during 1999-2008.(11) This validation study made use of prenatal records requested for all 3800 preeclamptic pregnancies that were part of the Norwegian Mother and Child Cohort Study (MoBa), a national birth cohort of 113,000 pregnancies recruited early in pregnancy during 1999-2008.(12) After attrition imposed by non-response, inadequate records, and our strict criteria for defining first diagnosis (see end of paragraph and Appendix 1, available online at http://links.lww.com/xxx), we could assign a week of diagnosis for 1857 (61%) of those preeclamptic pregnancies. We used this subset of 1857 to estimate the distribution of timing of preeclampsia diagnosis for all 21,020 preeclamptic pregnancies in the registry during the corresponding ten-year period. To receive a diagnosis of preeclampsia, both hypertension (systolic blood pressure ≥140mmHg or diastolic blood pressure ≥90mmHg) and proteinuria (≥1+ protein) had to be present at the same visit.(7) These criteria reflect the clinical definition of preeclampsia during the years of data collection.
Clinical diagnosis strictly requires that hypertension be documented twice. However, women with rapidly emerging symptoms may be transferred directly from the antenatal site to the hospital for confirmation of the disease, so that two measures may not be present in prenatal records for true cases. All cases in the subset received a diagnosis of preeclampsia in the Medical Birth Registry of Norway, suggesting that the criterion of a second measure had been met by the time of hospital discharge – even if documented only once in the antenatal records (which were limited to visits outside the hospital). Accordingly, we used the first visit where criteria were met as the gestational age of diagnosis.
Given that preeclampsia is frequently diagnosed at a routine prenatal clinic visit, even though signs of preeclampsia may have been present for some time during the interval since the previous prenatal visit, we defined the time of diagnosis, for purposes of analysis, as a time halfway between the prenatal visit of first diagnosis and the previous visit. Prenatal care in Norway is provided free of charge to all pregnant women and is widely attended,(13) which reduces potential bias from late entry into prenatal care or from infrequent care. In this population, women who eventually developed preeclampsia had a median of 2 weeks between visits until 30 weeks gestation and 1 week between visits after 30 weeks. Details on data collection, definitions, and exclusions are provided in Appendix 1 (http://links.lww.com/xxx).
The subset of women with known time of preeclampsia diagnosis provided a distribution of preeclampsia cases diagnosed in each gestational week, which we then applied to the larger sample of 21,020 preeclamptic pregnancies in the registry to determine the number of new pregnancies at risk each week for the whole population. An example of this calculation can be found in Table 1 (Column D).
Table 1.
Life Table values for calculation of weekly stillbirth risk in pregnancies with and without preeclampsia (PE) from 554,333 singleton births in the Norwegian Birth Registry 1999-2008
| Aa | Bb | Cc | Dd | Ee | Ff | Gg | Hh | Ii | Jj | Kk | Ll | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pregnancies at beginning of week | New Preeclampsia Cases |
Live Births during week | Stillbirths during week | Pregnancies at risk of stillbirth |
||||||||
| Week | All | With Preeclampsia |
Without Preeclampsia |
All | With Preeclampsia |
Without Preeclampsia |
All | With Preeclampsia |
Without Preeclampsia |
With Preeclampsia |
Without Preeclampsia |
|
| 23 | 362 | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| 24 | 554333 | 362 | 553971 | 158 | 222 | 22 | 200 | 135 | 5 | 130 | 430 | 553792 |
| 25 | 553976 | 494 | 553482 | 192 | 254 | 47 | 207 | 78 | 6 | 72 | 566 | 553283 |
| 26 | 553644 | 633 | 553011 | 204 | 319 | 78 | 241 | 75 | 7 | 68 | 696 | 552789 |
| 27 | 553250 | 752 | 552498 | 362 | 411 | 111 | 300 | 100 | 13 | 87 | 877 | 552167 |
| 28 | 552739 | 990 | 551749 | 396 | 487 | 168 | 319 | 68 | 2 | 66 | 1104 | 551391 |
| 29 | 552184 | 1216 | 550968 | 543 | 580 | 188 | 392 | 64 | 5 | 59 | 1394 | 550500 |
| 30 | 551540 | 1567 | 549973 | 815 | 796 | 233 | 563 | 70 | 5 | 65 | 1858 | 549284 |
| 31 | 550674 | 2144 | 548530 | 623 | 1020 | 301 | 719 | 73 | 7 | 66 | 2304 | 547860 |
| 32 | 549581 | 2458 | 547123 | 962 | 1486 | 357 | 1129 | 83 | 7 | 76 | 2761 | 546077 |
| 33 | 548012 | 3056 | 544956 | 1166 | 2262 | 475 | 1787 | 79 | 7 | 72 | 3402 | 543479 |
| 34 | 545671 | 3740 | 541931 | 1777 | 3755 | 638 | 3117 | 86 | 5 | 81 | 4310 | 539484 |
| 35 | 541830 | 4874 | 536956 | 2128 | 6064 | 905 | 5159 | 93 | 3 | 90 | 5486 | 533312 |
| 36 | 535673 | 6094 | 529579 | 2807 | 11783 | 1419 | 10364 | 116 | 5 | 111 | 6788 | 522993 |
| 37 | 523774 | 7478 | 516296 | 2502 | 26727 | 2179 | 24548 | 144 | 9 | 135 | 7639 | 502772 |
| 38 | 496903 | 7791 | 489112 | 2536 | 69905 | 3199 | 66706 | 182 | 6 | 176 | 7459 | 454491 |
| 39 | 426816 | 7122 | 419694 | 1958 | 126971 | 3827 | 123144 | 171 | 8 | 163 | 6187 | 357143 |
| 40 | 299674 | 5245 | 294429 | 1177 | 155226 | 3758 | 151468 | 198 | 4 | 194 | 3954 | 218107 |
| 41 | 144250 | 2660 | 141590 | 340 | 103758 | 2330 | 101428 | 132 | 5 | 127 | 1665 | 90706 |
| 42 | 40360 | 665 | 39695 | 11 | 40293 | 676 | 39617 | 67 | 0 | 67 | 332 | 19881 |
Additional details are in Appendix 2 (http://links.lww.com/xxx).
Column descriptions including definition and numeric example for week 28:
All ongoing pregnancies at the beginning of the week. Total number of pregnancies observed – Sum of all still and live births in previous weeks. For week 28: 554,333-(1206+388)=552,739.
Ongoing pregnancies with preeclampsia at the beginning of the week. Ongoing pregnancies with preeclampsia at the beginning of the previous week + New preeclampsia cases from the previous week – Live and still births with preeclampsia in the previous week. For week 28: 752 + 362 – 111 – 13 = 990.
Ongoing pregnancies without preeclampsia at the beginning of the week. Ongoing pregnancies without preeclampsia at the beginning of the previous week - New preeclampsia cases from the previous week – Live and still births without preeclampsia in the previous week. For week 28: 552,498 – 362 – 300 - 87 = 551,749.
New preeclampsia cases. Proportion of new preeclampsia cases diagnosed in this week observed in the subset and applied to all preeclampsia cases in the Registry. For week 28: (35/1857)*21,020 = 396.
Live and still births (total, with and without preeclampsia) observed in the Registry.
Live and still births (total, with and without preeclampsia) observed in the Registry.
Live and still births (total, with and without preeclampsia) observed in the Registry.
Live and still births (total, with and without preeclampsia) observed in the Registry.
Live and still births (total, with and without preeclampsia) observed in the Registry.
Live and still births (total, with and without preeclampsia) observed in the Registry.
Pregnancies with preeclampsia at risk of stillbirth. Ongoing pregnancies with preeclampsia at the beginning of the week + ½(New preeclampsia cases for the week) – ½(Live births with preeclampsia for the week). For week 28: 990 + (396/2) - (168/2) = 1104.
Pregnancies without preeclampsia at risk of stillbirth. Ongoing pregnancies without preeclampsia at the beginning of the week - ½(New preeclampsia cases for the week) – ½(Live births without preeclampsia for the week). For week 28: 551,749 - (396/2) - (319/2) = 551,391.
The risk of stillbirth in a specific week of pregnancy is often expressed as a proportion of the number of births in a specific week.(14) Such calculations do not express the risk in terms of the population actually at risk, namely all fetuses at that gestational age. Instead, we calculate the weekly risk of fetal death as a proportion of all fetuses (all pregnancies) in that week, which is the true population at risk. We constructed a life table enumerating pregnancies with and without preeclampsia at the beginning of each gestational week. New cases of preeclampsia (i.e., those projected to occur each week based on the distribution of diagnosis in the subset) are transferred from the unexposed risk-set to the preeclampsia risk-set. Deliveries are removed each week from their respective risk-sets (both stillbirths and live births). The full life table with examples of the calculations used is provided in Table 1.
From this life table we estimated the number of ongoing preeclamptic pregnancies in each week, which we then used to estimate the week-specific risk of fetal deaths in pregnancies with and without preeclampsia. We smoothed the mortality data using a three-week moving average (geometric means), and calculated relative risks from the smoothed data. Confidence intervals were estimated using resampling to incorporate the variability in the estimated distribution of preeclampsia diagnosis.(15) We provide a full description of the analytic methods in Appendix 2, available online at http://links.lww.com/xxx.
RESULTS
There were 554,333 eligible singleton pregnancies delivered in Norway in 1999-2008, of which 3.8% (n=21,020) had preeclampsia recorded at delivery. Maternal and fetal characteristics of pregnancies with and without preeclampsia are presented in Table 2. Maternal age was similar in the two groups. Preeclamptic women were slightly less likely to be smokers and more likely to be nulliparous, as commonly seen in other studies.(16)
TABLE 2.
Characteristics of 554,333 women and their infants delivered in Norway in 1999-2008, by presence of preeclampsia, together with the subset of 1857 women who provided data on exact date of diagnosis of preeclampsia
| Norwegian Medical Birth Registry | Validation Subseta | |||||
|---|---|---|---|---|---|---|
| No preeclampsia | Preeclampsia | Dated Preeclampsia | ||||
| Total births | 533313 | 21020 | 1857 | |||
| Stillbirth | 1905 | 109 | 7 | |||
| Stillbirth rate per 1,000 births | 3.6 | 5.2 | 3.8 | |||
| Characteristic | N | % | N | % | N | % |
| Maternal age | ||||||
| <=24 | 91552 | 17 | 4492 | 21 | 274 | 15 |
| 25-34 | 354307 | 66 | 13228 | 63 | 1275 | 69 |
| 35+ | 87416 | 16 | 3299 | 16 | 308 | 17 |
| Missing | 38 | 1 | 0 | |||
| Parity | ||||||
| 0 | 214336 | 40 | 12618 | 60 | 1219 | 66 |
| 1 | 192428 | 36 | 5224 | 25 | 413 | 22 |
| 2+ | 126549 | 24 | 3178 | 15 | 225 | 12 |
| Smoking at end of pregnancy | ||||||
| No | 360795 | 68 | 14856 | 71 | 1411 | 76 |
| Yes | 56086 | 11 | 1490 | 7 | 61 | 3 |
| Missing | 116432 | 22 | 4674 | 22 | 385 | 21 |
| Gestational Age at birth (week) | ||||||
| ≤26 | 918 | 0. 2 | 165 | 1 | 10 | 1 |
| 27-30 | 1851 | 0.4 | 725 | 3 | 50 | 3 |
| 31-34 | 7047 | 1 | 1797 | 9 | 133 | 7 |
| 35-36 | 15724 | 3 | 2332 | 11 | 213 | 11 |
| 37-38 | 91565 | 17 | 5393 | 26 | 487 | 26 |
| 39-40 | 274969 | 52 | 7597 | 36 | 685 | 37 |
| 41-42 | 141239 | 26 | 3011 | 14 | 279 | 15 |
| Birth weight (g) | ||||||
| <1000 | 1289 | 0. 2 | 491 | 2 | 27 | 1 |
| 1000-1999 | 4396 | 1 | 1893 | 9 | 138 | 7 |
| 2000-2999 | 59891 | 11 | 5419 | 26 | 504 | 27 |
| 3000-3999 | 354388 | 66 | 10051 | 48 | 930 | 50 |
| 4000+ | 112682 | 21 | 3140 | 15 | 258 | 14 |
| Missing | 667 | 0.1 | 26 | 0.1 | 0 | |
| Timing of fetal death | ||||||
| Before onset of labor | 1522 | 80 | 88 | 81 | 6 | 86 |
| During delivery | 131 | 7 | 6 | 6 | 0 | |
| Unknown | 252 | 13 | 15 | 14 | 1 | 14 |
| Neonatal Deathb | 843 | 0.16 | 71 0.34 | 2 | 0.11 | |
Subset of pregnancies with preeclampsia identified in the Norwegian Medical Birth Registry and with an identified date of diagnosis in a validation study using prenatal records.
Deaths in first 28 days following birth expressed per 100 live births.
The subset of 1857 pregnancies with known week of preeclampsia diagnosis were similar to the total population with preeclampsia (Table 2) although, the subset had slightly more nulliparous women (66% versus 60%) and non-smoking women (76% versus 71%).
Adjusted for time between prenatal visits, 8% of preeclampsia cases had been diagnosed by the end of week 28, 36% by the end of week 34, and 71% by the end of week 37. Median diagnosis of preeclampsia was at 36 weeks, with 10th and 90th percentiles at 29.5 and 39.5 weeks.
The risk of stillbirth was 3.6/1000 overall, and 5.2/1000 among pregnancies with preeclampsia (relative risk (RR) =1.45, 95% confidence interval (CI) =1.20 to 1.76). In pregnancies with no preeclampsia, the weekly risk of fetal death was extremely low – on the order of 0.1 to 0.9 deaths per 1000 pregnancies per week up to 40 weeks (Figure 1). In contrast, the risk of fetal death among pregnancies with preeclampsia was 11.6 per 1000 in week 26, 4.6 per 1000 in week 28, and 2.5 per 1000 in week 32. The corresponding relative risks are 86 in week 26, 36 in week 28, and 19 in week 32 (Table 3). All confidence intervals excluded the null expectation by a wide margin. A stratified analysis of first births using the distribution of preeclampsia diagnosis observed among first births in the subset, resulted in a very similar magnitude and pattern of relative risk (Appendix 3, available online at http://links.lww.com/xxx).
Figure 1. Per-week risk of fetal death for pregnancies with and without preeclampsia.
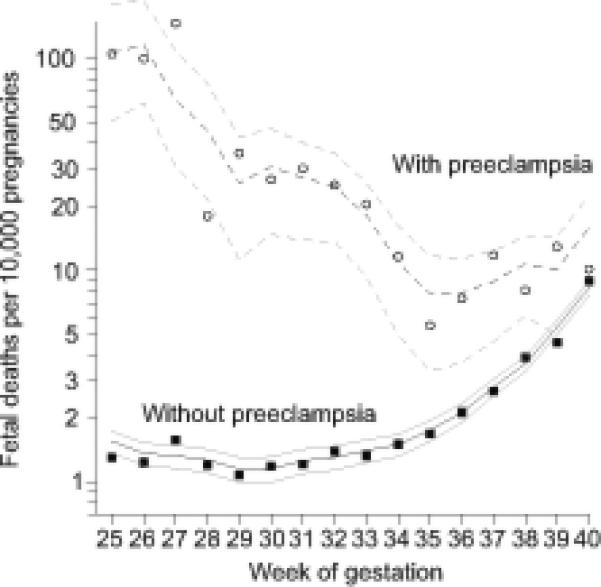
Circles and filled squares represent exact risks; darker lines represent three-week moving averages; pairs of lighter lines represent 95th percentile intervals.
Table 3.
Relative risk of fetal death in the presence of preeclampsia in 554,333 singleton pregnancies from Norway 1999-2008
| Smootheda week-specific risk of fetal death per 1,000 ongoing pregnancies |
||||
|---|---|---|---|---|
| Week | Preeclampsia | No Preeclampsia | Relative Risk | 95% Confidence Intervalb |
| 25 | 10.7 | 0.16 | 69 | 33 to 120 |
| 26 | 11.6 | 0.14 | 86 | 46 to 142 |
| 27 | 6.5 | 0.13 | 49 | 24 to 83 |
| 28 | 4.6 | 0.13 | 36 | 17 to 61 |
| 29 | 2.6 | 0.11 | 23 | 10 to 38 |
| 30 | 3.1 | 0.12 | 27 | 13 to 42 |
| 31 | 2.7 | 0.13 | 22 | 11 to 33 |
| 32 | 2.5 | 0.13 | 19 | 10 to 28 |
| 33 | 1.8 | 0.14 | 13 | 6.5 to 19 |
| 34 | 1.1 | 0.15 | 7.3 | 3.3 to 11 |
| 35 | 0.8 | 0.18 | 4.4 | 1.9 to 6.8 |
| 36 | 0.8 | 0.21 | 3.7 | 1.7 to 5.4 |
| 37 | 0.9 | 0.28 | 3.2 | 1.6 to 4.4 |
| 38 | 1.1 | 0.36 | 3.0 | 1.7 to 4.1 |
| 39 | 1.0 | 0.54 | 1.9 | 0.9 to 2.7 |
| 40 | 1.6 | 0.83 | 1.9 | 0.9 to 2.7 |
Smoothed using a 3-week running geometric mean
95% bootstrap percentile confidence intervals based on 10,000 resamples of the time-of-diagnosis distribution and both live and stillbirth distributions conditional on preeclampsia status
Our estimates of fetal risk depend on the accurate timing of preeclampsia diagnosis (derived from prenatal records). Any error that underestimates the proportion with early-onset preeclampsia would reduce the denominator in a given preterm week and thus inflate fetal risk. Similarly, overestimating the proportion with early onset would underestimate early fetal risk.
Our estimate of time of preeclampsia diagnosis excluded pregnancies that did not meet our clinical definition of preeclampsia based on prenatal records (i.e., before being admitted to hospital for delivery). By default, such exclusion assumes those pregnancies had the same average time of diagnosis as other preeclamptic pregnancies. In a sensitivity analysis, we made the extreme alternative assumption, that preeclampsia in these pregnancies emerged as late as possible (i.e. on the day of delivery). As expected, this shift to diagnosis in later weeks reduced the estimated prevalence of preeclampsia in earlier weeks and increased the estimated fetal risk with preterm preeclampsia (see Appendix 1 and Appendixes #4 and #5, all available online at http://links.lww.com/xxx, for detailed methods and results).
DISCUSSION
Clinicians are aware of the increased risk of fetal death among pregnancies diagnosed with preeclampsia in the preterm period. Efforts to quantify this risk, however, have paradoxically suggested that highest relative risk of fetal death with preeclampsia is during the term period.(1,4-6) We address this question in a novel way, by estimating the risk of fetal death at each gestational week given the estimated presence (or absence) of preeclampsia in that week. While the baseline risk of fetal death in a given week is low, fetal risk with preeclampsia was 86-fold higher in week 26, almost 50-fold higher in week 27, and more than 35-fold higher in week 28. Even in week 34, fetal risk was increased more than 7-fold. This elevated fetal risk is plausibly due to the disorders of placental function that cause preeclampsia,(17) or to systemic maternal responses to inadequate placentation.
The week-specific risk of fetal death with early preeclampsia is difficult to estimate for at least three reasons. First, very large study populations are required. The exposure and outcome are both rare, and the absolute risk remains small. To accurately measure risk, we assembled data on all Norwegian births over a 10-year period – and even then, estimates within gestational-age strata were limited by small numbers.
A second obstacle to the estimation of fetal risk with preeclampsia is the inaccessibility of information on time of preeclampsia diagnosis. To assume that preeclampsia is present early in all pregnancies subsequently diagnosed would drastically underestimates fetal risk at early gestational ages by inflating the weekly population at risk. We were able to estimate time-ofpreeclampsia diagnosis by taking advantage of data from a special study of nearly nineteen hundred women with incident preeclampsia, a subset that could reasonably be extrapolated to the whole population of preeclamptic pregnancies.
A third issue in estimating fetal risk lies in the definition of fetal mortality. We defined fetal risk in relation to all fetuses present at a given gestational week. This approach is rational but (for historical reasons) not standard. The more common definition of stillbirth risk in vital statistics and elsewhere has been the number of stillbirths divided by the number of all births (stillbirths plus live births).(14) While this risk measure is informative when applied to the overall stillbirth rate, it has dubious clinical relevance when applied to specific gestational weeks. This problem has been recognized since at least 1987, when Yudkin and colleagues(18) suggested that the risk of death among all fetuses at a given gestational age is the more clinically relevant measure. Yudkin's definition has won acceptance in principle (19-22) and has recently appeared in US vital statistics reports,(23) but has not yet been widely applied.
The standard definition of stillbirth rate has another (if more subtle) disadvantage: it is vulnerable to strong bias in the presence of unmeasured factors that cause both preterm delivery and stillbirth.(24,25) Such unmeasured factors become concentrated in non-preeclamptic preterm births, making stillbirth appear higher in non-preeclamptic than preeclamptic pregnancies.(1,16) This apparent “protective effect” of preeclampsia during the preterm weeks has sometimes been misinterpreted as evidence that preeclampsia biologically reduces fetal risk during the preterm weeks.(26) Our results show that the opposite is true – preterm preeclampsia constitutes a serious threat to the fetus.
Management of severe preeclampsia involves balancing the welfare of the mother and the fetus. There is a further dilemma with regard to the fetus, in that early delivery spares further risk from fetal death but exposes the preterm infant to the dangers of neonatal morbidity and mortality. A recent Cochrane review(27) assessed the fetal consequences of immediate versus delayed delivery in pregnancies with “severe preeclampsia” (before 34 weeks). Net survival of the fetus (fetal plus neonatal mortality) was similar with immediate or delayed delivery (risk ratio with immediate delivery 1.08 (0.69 to 1.71)). While our data may help to further quantify fetal risk among women diagnosed with preeclampsia, clinical decision-making will continue to depend on clinical judgment and the specific clinical picture of each mother-and-fetus pair.
Our assessment of fetal risk with preterm preeclampsia was made possible by combining data from the Norwegian birth registry with a smaller sample of detailed antenatal charts. These two resources combine the strength of population-level data on stillbirths with detailed clinical data on the timing of preeclampsia diagnosis for a substantial subset. Analyzing these data with a fetuses-at-risk approach(18) quantified a hazard for fetuses in preterm preeclamptic pregnancies. The same approach could equally apply to assessment of fetal risk with any condition that emerges during pregnancy and persists.
The study has important limitations. One, preeclampsia is incompletely captured by the Medical Birth Registry of Norway.(11) Unrecorded cases of preeclampsia, misclassified as “non-cases” in our analysis, would tend to reduce our estimates of fetal relative risk. A more serious error would be false-positive diagnoses of preeclampsia in the birth registry. However, the positive predictive value of preeclampsia registration in the Medical Birth Registry of Norway has been estimated at 85% overall and 94% in preterm births.(11) Indicators of severity of disease are less reliably recorded.(11) In particular fetal growth restriction at the time of diagnosis is not available in the registry and precludes analysis among these particularly vulnerable fetuses.
Another limitation is sample size. Even with data from a half-million births, the low rates of fetal mortality in Norway produce relatively few stillbirths. It would have been informative to stratify our analysis by maternal parity or smoking, but estimates of fetal mortality were much less stable in those smaller strata.
The Medical Birth Registry of Norway lacks information on obesity and other maternal factors that might confound analyses of preeclampsia and stillbirth. Given that our main finding was a strong gradient of risk across gestational age, it is implausible that adjustment for maternal characteristics that are stable across gestational age would alter that conclusion.
There are urgent clinical questions that these data cannot address. Both severity and duration of preeclampsia could reasonably be expected to affect the level of fetal risk. The birth registry lacks dates of preeclampsia diagnosis and specific features of severe disease at the time of diagnosis. Our estimates provide simply the average risk among all preeclamptic pregnancies at given gestational weeks.
In sum, our analysis documents the fetal risk that accompanies preeclampsia in early pregnancy. While this risk to the fetus is generally recognized, the extent of risk is far higher than previously estimated.
Supplementary Material
Précis.
There is a remarkably high relative risk of stillbirth among pregnancies diagnosed with preeclampsia in the preterm period.
Acknowledgments
The Norwegian Mother and Child Cohort Study (MoBa) is supported by the Norwegian Ministry of Health and the Ministry of Education and Research, NIH/NIEHS (contract no N01-ES-75558), NIH/NINDS (grant no.1 UO1 NS 047537-01, grant no.2 UO1 NS 047537-06A1), and the Norwegian Research Council/ FUGE (grant no. 151918/S10). The validation of preeclampsia diagnoses was funded by the National Institute of Child Health and Human Development (R01HD058008, PI Engel). This research was supported in part (Allen J. Wilcox, David M. Umbach, Quaker E. Harmon) by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences (Z01 ES044003).
The authors thank Drs. Donna Baird, Curt Eshelman, David Grimes, Ellen Harrison, James Roberts, Dale Sandler, and Clarice Weinberg for comments on earlier drafts of this manuscript. Dr. D. Robert McConnaughey made the figures, and Dr. Marta Ebbing kindly provided updated data on stillbirths from the Norwegian Medical Birth Registry.
Footnotes
Financial Disclosure: The authors did not report any potential conflicts of interest.
Presented at the 2014 annual meetings of the Society for Pediatric and Perinatal Epidemiologic Research (June 23-24) and the Society for Epidemiologic Research (June 24-27, Seattle, WA).
References
- 1.Ahmad AS, Samuelsen SO. Hypertensive disorders in pregnancy and fetal death at different gestational lengths: a population study of 2 121 371 pregnancies. BJOG. 2012;119:1521–8. doi: 10.1111/j.1471-0528.2012.03460.x. [DOI] [PubMed] [Google Scholar]
- 2.Ananth C, Basso O. Impact of pregnancy-induced hypertension on stillbirth and neonatal mortality. Epidemiology. 2010;21:118–23. doi: 10.1097/EDE.0b013e3181c297af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet (London, England) 2005;365:785–99. doi: 10.1016/S0140-6736(05)17987-2. [DOI] [PubMed] [Google Scholar]
- 4.Ananth CV, Savitz DA, Bowes WA. Hypertensive disorders of pregnancy and stillbirth in North Carolina, 1988 to 1991. Acta Obstet Gynecol Scand. 1995;74:788–93. doi: 10.3109/00016349509021198. [DOI] [PubMed] [Google Scholar]
- 5.Smulian J, Ananth C, Vintzileos A, Scorza W, Knuppel R. Fetal deaths in the United States. Influence of high-risk conditions and implications for management. Obstet Gynecol. 2002;100:1183–9. doi: 10.1016/s0029-7844(02)02389-x. [DOI] [PubMed] [Google Scholar]
- 6.Piper JM, Langer O, Xenakis EM, McFarland M, Elliott BD, Berkus MD. Perinatal outcome in growth-restricted fetuses: do hypertensive and normotensive pregnancies differ? Obstet Gynecol. 1996;88:194–9. doi: 10.1016/0029-7844(96)02169-2. [DOI] [PubMed] [Google Scholar]
- 7.ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. Obstetrics and gynecology. 2002;99:159–67. doi: 10.1016/s0029-7844(01)01747-1. [DOI] [PubMed] [Google Scholar]
- 8.Irgens LM. The Medical Birth Registry of Norway. Epidemiological research and surveillance throughout 30 years. Acta Obstet Gynecol Scand. 2000;79:435–9. [PubMed] [Google Scholar]
- 9.Backe B. [Routine ultrasonography in obstetric care in Norway, 1994]. Tidsskrift for den Norske lægeforening. 1997;117:2314–5. [PubMed] [Google Scholar]
- 10.Skjærven R, Gjessing HK, Bakketeig LS. Birthweight by gestational age in Norway. Acta obstetricia et gynecologica Scandinavica. 2000;79:440–9. [PubMed] [Google Scholar]
- 11.Klungsøyr K, Harmon QE, Skard L, Simonsen I, Austvoll E, Starling AP, et al. Validity of preeclampsia registration in the Medical Birth Registry of Norway for women participating in the Norwegian Mother and Child Cohort Study, 1999-2010. Paediatr Perinat Epidemiol. 2014 doi: 10.1111/ppe.12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schreuder P, Alsaker E. The Norwegian Mother and Child Cohort Study (MoBa)--MoBa recruitment and logistics. Norsk Epidemiologi. 2014:24. doi: 10.5324/nje.v24i1-2.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delvaux T, Buekens P. Disparity in prenatal care in Europe. Study group on barriers and incentives to prenatal care in Europe. European journal of obstetrics & gynecology and reproductive biology. 1999;83:185–90. doi: 10.1016/s0301-2115(98)00237-1. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen RHN, Wilcox A. Terms in reproductive and perinatal epidemiology: I. Reproductive terms. J Epidemiol Community Health. 2005;59:916–9. doi: 10.1136/jech.2004.023457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Efron B. An introduction to the bootstrap. Chapman & Hall/CRC; Boca Raton: 1993. [Google Scholar]
- 16.Hutcheon J, Lisonkova S, Joseph KS. Epidemiology of pre-eclampsia and the other hypertensive disorders of pregnancy. Best practice & research. Clinical obstetrics & gynaecology. 2011;25:391–403. doi: 10.1016/j.bpobgyn.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 17.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005 Jun;308:1592–4. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- 18.Yudkin PL, Wood L, Redman CW. Risk of unexplained stillbirth at different gestational ages. Lancet (London, England) 1987;1:1192–4. doi: 10.1016/s0140-6736(87)92154-4. [DOI] [PubMed] [Google Scholar]
- 19.Joseph KS, Kramer MS, Allen AC, Cyr M, Fair M, Ohlsson A, et al. Gestational age-and birthweight-specific declines in infant mortality in Canada, 1985-94. Fetal and Infant Health Study Group of the Canadian Perinatal Surveillance System. Paediatr Perinat Epidemiol. 2000;14:332–9. doi: 10.1046/j.1365-3016.2000.00298.x. [DOI] [PubMed] [Google Scholar]
- 20.Wilcox A, Weinberg C. Invited commentary: analysis of gestational-age-specific mortality--on what biologic foundations? Am J Epidemiol. 2004;160:213–4. doi: 10.1093/aje/kwh204. [DOI] [PubMed] [Google Scholar]
- 21.Paneth N. Stillbirth: still important and still a puzzle. Epidemiology. 2012;23:255–6. doi: 10.1097/EDE.0b013e3182461056. [DOI] [PubMed] [Google Scholar]
- 22.Auger N, Delézire P, Harper S, Platt R. Maternal education and stillbirth: estimating gestational-age-specific and cause-specific associations. Epidemiology. 2012;23:247–54. doi: 10.1097/EDE.0b013e31824587bc. [DOI] [PubMed] [Google Scholar]
- 23.MacDorman MF, Kirmeyer SE, Wilson EC. National vital statistics reports. Vol. 60. National Center for Health Statistics; Hyattsville, MD: 2012. Fetal and perinatal mortality, United States 2006. [PubMed] [Google Scholar]
- 24.Wilcox A, Weinberg C, Basso O. On the pitfalls of adjusting for gestational age at birth. Am J Epidemiol. 2011;174:1062–8. doi: 10.1093/aje/kwr230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.VanderWeele T, Mumford S, Schisterman E. Conditioning on intermediates in perinatal epidemiology. Epidemiology. 2012;23:1–9. doi: 10.1097/EDE.0b013e31823aca5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Facchinetti F, Alberico S, Benedetto C, Cetin I, Cozzolino S, Di Renzo G, et al. A multicenter, case-control study on risk factors for antepartum stillbirth. Journal of Maternal -Fetal & Neonatal Medicine. 2011;24:407–10. doi: 10.3109/14767058.2010.496880. [DOI] [PubMed] [Google Scholar]
- 27.Churchill D, Duley L, Thornton JG, Jones L. Interventionist versus expectant care for severe pre-eclampsia between 24 and 34 weeks' gestation. Cochrane Database of Systematic Reviews. 2013 doi: 10.1002/14651858.CD003106.pub2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


