Abstract
Objective
We hypothesize that prenatal exposure to glucocorticoids (GCs) will negatively alter the insulin signal transduction pathway and has differing effects on the fetus according to gestational age at exposure.
Methods
Twenty-three fetal baboons were delivered from twenty-three healthy, non-diabetic mothers. Twelve preterm (0.67 gestational age) and eleven near term (0.95 gestational age) baboons were euthanized immediately after delivery. Half of the pregnant baboons at each gestation received two doses of intramuscular betamethasone 24-hours apart (170 μg.kg−1) before delivery, while the other half received no intervention. Vastus lateralis muscle was obtained from postnatal animals to measure protein content and gene expression of insulin receptor (IR)-β, IR-β Tyr 1361 phosphorylation (pIR-β), IR substate-1 (IRS-1), IRS-1 tyrosine phosphorylation (pIRS-1), p85 subunit of PI3-kinase (p85), Akt (Protein Kinase B), phospho-Akt Ser473 (pAkt), Akt-1, Akt-2, and glucose transporters (GLUT1 and GLUT4).
Results
Skeletal muscle from preterm baboons exposed to glucocorticoids had markedly reduced protein content of Akt and Akt-1 (respectively, 73% and 72% from 0.67 gestational age Control, P<0.001); IR-β and pIR-β were decreased (respectively, 94% and 85%, P<0.01) in the muscle of premature GC exposed fetuses, but not in term fetuses. GLUT1 and GLUT4 tended to increase with GC exposure in preterm animals (P=0.09), while GLUT4 increased 6.0 fold in term animals after GC exposure (P<0.05).
Conclusion
Exposure to a single course of antenatal GCs during fetal life alters the insulin-signaling pathway in fetal muscle in a manner dependent on the stage of gestation.
Keywords: insulin, fetus, primate, muscle, glucocorticoid, corticosteroid, preterm
INTRODUCTION
Antenatal corticosteroid administration to pregnant women has become the standard of care for mothers at risk for delivering a premature infant. Maternal treatment with synthetic glucocorticoids (GCs) decreases the incidence of many morbidities associated with prematurity, including respiratory distress syndrome and intraventricular hemorrhage (“Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consensus Development Panel on the Effect of Corticosteroids for Fetal Maturation on Perinatal Outcomes” 1995, Brownfoot et al. 2008). Short-term adverse effects seen with postnatal administration of steroids include hypertension, hyperglycemia, and growth restriction (Ng, 1993). Furthermore, animal and human studies have shown that GC use during the fetal period may increase the likelihood of acquiring adult diseases earlier in life, more specifically hypertension, coronary heart disease, and diabetes (Mizuno et al. 2013, Newnham 2001). Moreover, GC exposure during pregnancy impacts placental growth factors and insulin-signaling pathways during late gestation (Ain 2005, Jellyman et al. 2012). In the fetal sheep, GLUT4 was increased in skeletal muscle when GCs were administered in late gestation, whereas Akt (Protein Kinase B) and mTOR pathway molecules were only increased in the muscle of those fetuses exposed to cortisol infusion but not GCs (Jellyman et al. 2012).
Extremely low birth weight (ELBW) infants (< 1000 gram birth weight) have a high incidence of hyperglycemia (Beardsall et al. 2010, Blanco et al. 2006, Liechty 2010), relative insulin resistance, and defective insulin processing; however, the molecular basis for their impaired glucose metabolism remains unclear (Mitanchez-Mokhtari et al. 2004). In human fetuses, insulin promotes skeletal muscle protein synthesis and growth, which peak by the second trimester, and both synthesis and growth decrease progressively thereafter. Variations in insulin concentrations during intrauterine life contribute to differences in fetal growth (Fowden et al. 1989, Philipps et al. 1991, Schwartz & Teramo 2000). Insulin must bind to its receptor and initiate the insulin-signaling cascade through the insulin receptor (IR) and IR substrate-1 (IRS-1), which is followed by activation of Akt. Insulin-responsive tissues (i.e., skeletal muscle) are enriched in Akt, which is considered a key mediator of both cell growth and metabolism (Calera et al. 1998, Cho et al. 2001). We have previously demonstrated a significantly increased content of IR, total Akt, and Akt isoforms in fetal skeletal muscle of 125 day (d) gestational age (GA) preterm baboons (0.67 gestation) when compared to term baboons (Blanco et al. 2010). These increased protein levels in immature animals are likely related to the accelerated growth that occurs in mid-gestation compared to the period just prior to delivery when differentiation of organs for postnatal function takes precedence over growth and proliferation. Previous studies have shown decreased levels of GLUT1 and GLUT4 in skeletal muscle of fetal preterm baboons (Blanco et al. 2010); these differences might be developmental as glucose homeostasis is controlled by passive diffusion through the placenta during fetal life and insulin stimulated glucose disposal is decreased in-utero. Others have shown perturbations in fetal skeletal muscle glucose transporters in sheep after a single dose of GCs, along with fetal hyperglycemia and hyperinsulinemia (Gray et al. 2006). Therefore, information is needed on the precise change in insulin function and signaling at the muscle cell level resulting from fetal exposure to concentrations of corticosteroids in excess of those appropriate for the current stage of maturation.
Our previous studies have shown that the baboon is a valuable animal model in which to study insulin-signaling. Baboons have 97% phylogenetic proximity with humans and spontaneously develop insulin resistance when obese (Blanco et al. 2013; Chavez et al. 2008). Additionally, baboons develop common complications pertinent to preterm infants, such as glucose abnormalities, bronchopulmonary dysplasia, and patent ductus arteriosus (Escobedo et al. 1982, McCurnin & Clyman 2008). Preterm baboons also have an incidence of hyperglycemia comparable to ELBW neonates and heightened differences in the insulin-signaling pathway when compared to term primates (Blanco et al. 2010, 2013).
We hypothesize that prenatal exposure to GCs reduces the content of key signaling molecules in the insulin signal transduction pathway in a manner dependent on the stage of gestation with differing effects on preterm baboons and term baboons. In this study, we examined effects of antenatal GC exposure on the insulin-signaling proteins of skeletal muscle in fetal baboons delivered extremely premature and at term.
MATERIALS AND METHODS
Animal maintenance
All animals were obtained from the Southwest National Primate Research Center at the Southwest Foundation for Biomedical Research (SFBR) in San Antonio, Texas. Studies were approved by the Institutional Animal Care Committee at the SFBR and conducted in accordance with standard, humane animal care. Twenty-three fetal baboons (Papio hamadryas, 18 males, 5 females) were delivered prematurely at 125 d GA (n=12) or 175d-190d GA (n=11; full term is 185d GA) under general anesthesia via C-section, as previously described in detail (Escobedo et al. 1982, McCurnin & Clyman 2008), or vaginally (term animals only) from 23 healthy, non-diabetic mothers. Half of the pregnant animals at each of the two designated stages of gestation received no intervention (controls=CTR) and half received two intramuscular (IM) betamethasone doses (170 μg.kg−1) 24-hours apart, with the second dose 24-hours prior to delivery. Based on our previous findings, GLUT1 protein content in skeletal muscle of preterm baboons increases from 33% to 99% when compared to term baboons; with a similar increase after GC exposure in preterm baboons, a 5% significance level and 80% power, a sample size of 5 animals per group was calculated.
Animals were euthanized shortly after birth with 1ml/10 lbs of intravenous pentobarbital, followed by exsanguination. Vastus lateralis muscle samples were immediately obtained, and tissues were promptly snap-frozen in liquid nitrogen and stored at − 80°C.
Measurement of insulin-signaling and glucose transporter proteins by Western blot analysis and immunoprecipitation
Primary antibodies against IR-β, pIR-β, and Akt-1 were purchased from Santa Cruz Biotechnology® (Santa Cruz, USA), IRS-1, p85 subunit of PI3-kinase (p85), Akt-2, GLUT1, and GLUT4 were from Millipore® (Chicago, USA), and GAPDH, pIR-β, phospho-Akt Ser473 (pAkt), and total Akt were obtained from Cell Signaling Technology® (Danvers, USA). Insulin-signaling molecules were measured in skeletal muscle using our previously described protocol (Blanco et al. 2010). The intensities of the bands were quantified by densitometry using the NIH imaging program with the results reported in arbitrary optical density (OD) units. GAPDH and/or Ponceau S from Thermo Fisher Scientific (Walthman, USA) were utilized as loading controls. For measurement of IRS-1 tyrosine phosphorylation (pIRS-1), proteins were immunoprecipitated with anti-IRS-1 antibody followed by incubation with protein A agarose beads as previously described (Blanco et al. 2010). The gels were normalized using internal controls to ensure comparable gel-to-gel data analysis across groups.
Statistical Analysis
Statistical calculations and demographic distributions were performed with SPSS for Microsoft Windows®, (Version 17.0, SPSS, Inc., Chicago, USA). Differences between groups were determined utilizing two-way ANOVA, followed by the Tukey test. A P<0.05 was considered to be statistically significant.
RESULTS
Animal characteristics
The 23 animals were delivered at two different gestations (125d GA and 185d GA). Animal characteristics are shown in Table 1.
TABLE 1.
Animal characteristics of fetal baboons used to investigate developmental differences of key insulin-signaling proteins in skeletal muscle.
| Group | Gestation (% of term) | n | Male/Female | Birth weight (g) |
|---|---|---|---|---|
| 125d CTR | 67 | 6 | 4/2 | 407 ± 47 |
| 125d GC | 67 | 6 | 5/1 | 352 ± 26 |
| Term CTR | 94 | 6 | 5/1 | 1008 ± 132 |
| Term GC | 94 | 5 | 4/1 | 1120 ± 134 |
d-day; CTR-control; GC-glucocorticoid; g-grams.
Content of IR-β, IRS-1, and p85 subunit of PI3-kinase
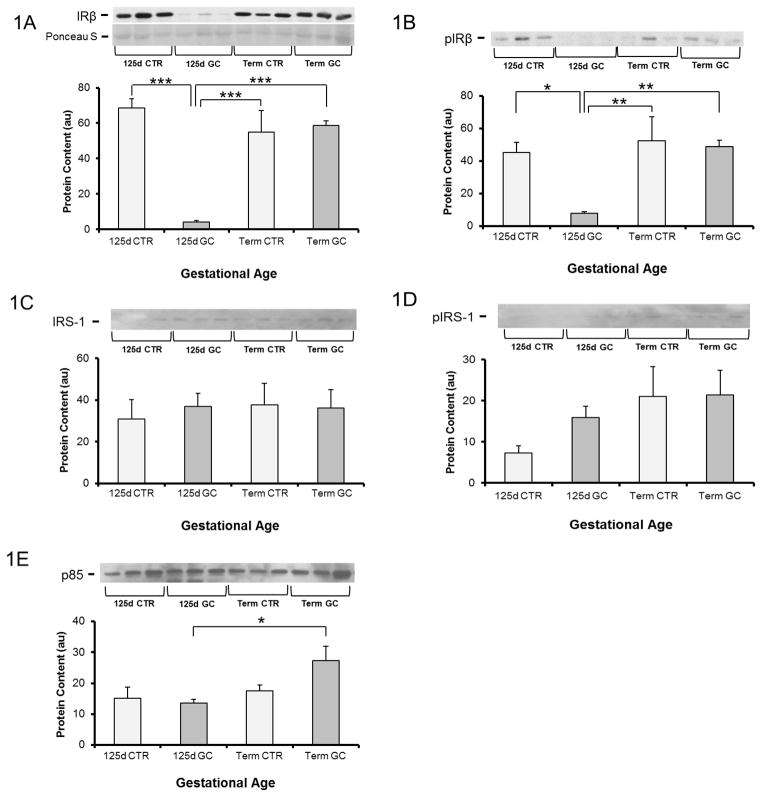
Figure 1A shows that the protein content of IR-β in muscle of preterm fetal baboons exposed to GCs was decreased by 94% compared to levels in preterm fetal baboons not exposed to GCs and was 93% less than term baboons exposed to GCs (P<0.001). No change in protein content was seen in term fetuses following GC exposure. Consistent with this finding, pIR-β was also decreased (by 85%) in the muscle of premature GC exposed fetuses, but not term fetuses (P<0.01) (Fig 1B). Muscle protein content of IRS-1 and pIRS-1, was similar across gestational ages regardless of antenatal GC exposure (Fig 1C–D). P85 was 2.0 fold higher in term baboons exposed to GCs, as compared to preterm baboons exposed to GC (Fig 1E).
Figure 1. Effect of glucocorticoid treatment (GC) on insulin signaling protein content and phosphorylation in muscle from fetal baboons at 0.67 gestation and term baboons.
Insulin receptor (IR)-β (A), IR-β Tyr 1361 phosphorylation (pIR-β) (B), IR substrate-1 (IRS-1) (C), IRS-1 tyrosine phosphorylation (pIRS-1) (D), and p85 subunit of PI 3-kinase (p85) (E) were measured by Western blotting and immunoprecipitation. n=5–6 per group. Representative blots from 3 animals per group are also shown. d-day; CTR-control; GC-glucocorticoid. Graphical data are means ± SE. *p<0.05, **p<0.01, ***p<0.001.
Muscle Akt content
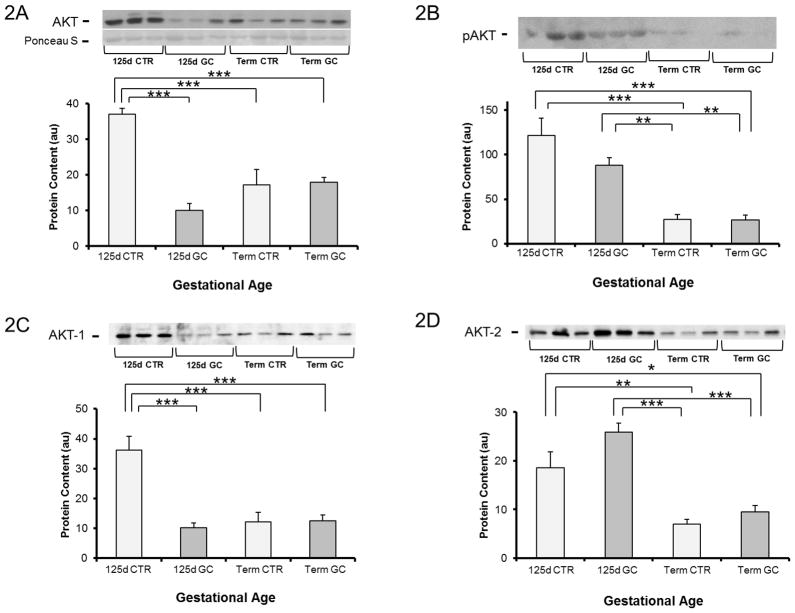
Baseline total Akt protein content was 54% greater in preterm fetal baboons versus term fetal baboons (P<0.01). When exposed to GCs, preterm baboons had a 73% decrease in Akt content (P<0.001), whereas no change was observed after GC administration in term animals (Fig 2A). In line with Akt protein content, pAkt was 4.5 fold higher in the muscle of preterm control fetuses compared to term controls (P<0.05). After GC exposure, protein content was decreased by 27% in preterm but not in term fetuses; however, it failed to reach statistical significance (P=0.2)(Fig 2B). Figure 2C shows that the decrease in Akt-1 observed in preterm animals exposed to GCs parallels the responses to GCs seen in total Akt and pAkt protein content. Akt-2 was 2.6 fold higher in immature fetal control baboons when compared to term controls; after GC exposure it tended to increase in preterm baboons (P=0.1), whereas no effect was seen after GC exposure in term baboons (Fig 2D).
Figure 2. Akt protein content and phosphorylation in muscle from fetal baboons at 0.67 gestation and term baboons.
Total Akt (Protein Kinase B) (A), phospho-Akt Ser473 (pAkt) (B), Akt-1 (C), and Akt-2 (D) were measured by Western blotting. n= 5–6 per group. Representative blots from 3 animals per group are also shown. d-day; CTR-control; GC-glucocorticoid. Graphical data are means ± SE. *p<0.05, **p<0.01, ***p<0.001.
Glucose transporter content in muscle
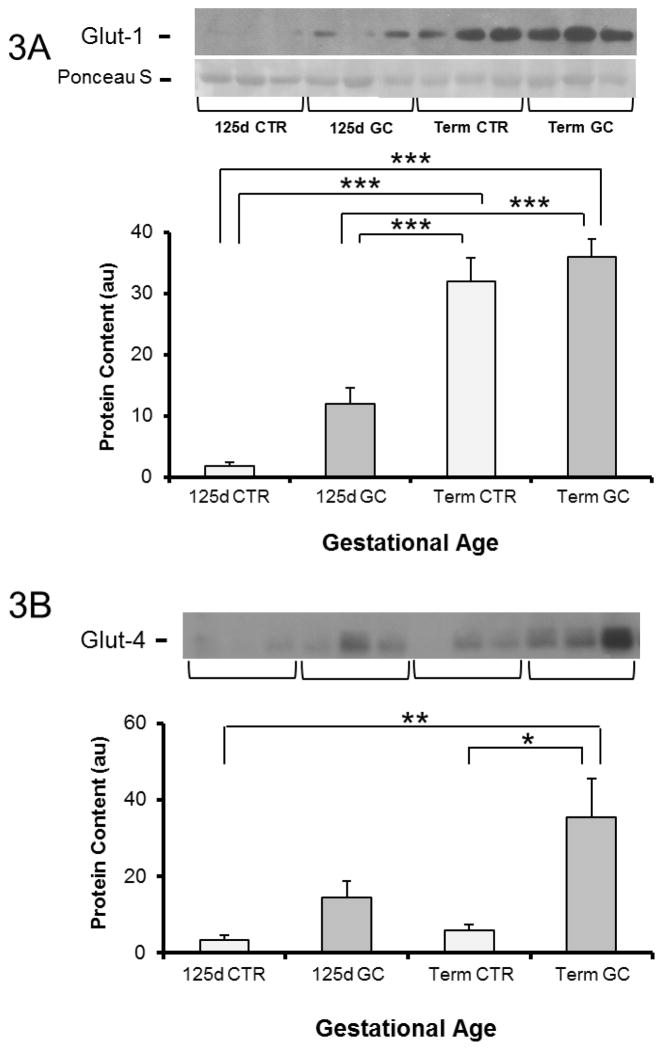
In contrast to IR-β, IRS-1 and Akt, the muscle of 125d GA fetal baboons had markedly lower GLUT1 protein content compared to term fetuses (P<0.001) (Fig 3A). There was a trend towards increased GLUT1 after GC exposure (P=0.09) in the preterm fetuses, whereas no changes were seen in term fetuses exposed to GCs. GLUT4 protein content increased in term fetuses by 6.0 fold after GC exposure (P<0.05) whereas only an increasing trend was observed in preterm fetuses after exposure to GCs (Fig 3B).
Figure 3. Glucose transporter protein content in muscle from fetal baboons at 0.67 gestation and term baboons.
GLUT1 (A) and GLUT4 (B) protein content. n= 5–6 per group. Representative blots from 3 animals per group are also shown. d-day; CTR-control; GC-glucocorticoid. Graphical data are means ± SE. *p<0.05, **p<0.01, *** p<0.001.
DISCUSSION
Treatment with GCs is widely used on women threatening premature delivery to reduce major neonatal morbidities. However, there are minimal data to assist in determining the appropriate dose, type, route, and potential differences in responses at different stages of fetal life. As with all powerful therapies, GCs can have short-term adverse effects in the fetal and postnatal period that can lead to alterations in-utero, resulting in life-long sequelae (Braun et al. 2009, Drake et al. 2011, Long et al. 2013a, Long et al. 2013b). The purpose of the present study was to examine the effects of fetal exposure to GCs on insulin-signaling pathways in skeletal muscle of preterm fetal baboons compared to term fetal baboons. Although antenatal GCs are not currently given in term pregnancies, they are given up to 85% gestation. The use of a well-established non-human primate model of fetal development aids translation to human fetal development.
We found significant differences in the effects of a single course of GCs on key insulin-signaling and glucose transporters in the muscle of fetal baboons. Importantly, some proximal signaling proteins in muscle are affected differently depending on gestational age at GC exposure. The most striking observation was the 94% reduction in the protein content of IR-β and 73% reduction in Akt protein content that occurred after a single course of GCs in preterm baboons, in contrast to the lack of any effect in term baboons. Akt and IR play other roles besides insulin-signaling. During embryonic development, insulin-like growth factors (IGFs) promote growth via two receptors, IR and the IGF-I receptor (Louvi et al. 1997). In human fetuses, variations in insulin concentrations contribute to alterations in fetal growth. Elevated insulin concentrations during uncontrolled maternal diabetes promote fetal overgrowth, while isolated fetal insulin deficiency under a stable maternal environment can cause fetal growth restriction (Fowden 1989, Philipps et al. 1991). Therefore, the significant decrease in IR and Akt content seen in immature animals exposed to one dose of GCs will likely stunt the normal acceleration in fetal growth that occurs during this critical period of development. Impairment of cell growth and proliferation will tend to restrict attainment of normal muscle mass and hence have potential major effects on life-time function including glucose metabolism. Skeletal muscle is the principal site for glucose utilization, and it is the primary tissue responsible for insulin resistance in obese and type 2 diabetic subjects (Lowell & Shulman 2005, Ozanne et al. 2005, Selak et al. 2003). Whether changes in skeletal muscle IR and Akt concentrations persist postnatally and translate into insulin resistance later in life remains to be determined.
Insulin-responsive tissues are enriched in Akt which is thought to be a key mediator of cell growth and metabolism (Kelly et al. 2012). Akt exists as three isoforms. In muscle, the most abundant are Akt-1 and Akt-2 (Datta et al. 1999, Walker et al. 1998). It has been proposed that Akt-1 is implicated in cell growth, whereas Akt-2 is thought to play a more important role in regulating glucose metabolism (Bae et al. 2003, Brozinick et al. 2003). We found a decrease in Akt-1 in preterm animals after exposure to GCs suggesting the major effect of GCs is on growth, whereas Akt-2 did not change after GC exposure. It is possible that transient changes in insulin-signaling protein content in the muscle of preterm baboons could be due to concurrent variations in fetal insulin and/or glucose concentrations after GC exposure (Jellyman et al. 2005, Grey et al. 2006). However, birth weights were similar within gestational groups and published plasma glucose and insulin levels measured at birth were similar in preterm and term control animals (Blanco et al. 2010). A limitation of this study is the lack of plasma glucose and insulin measurement in the GC treated animals.
Consistent with our previous studies (Blanco et al. 2010), we observed reduced GLUT1 content in immature baboons compared to term baboons. Both, GLUT1 and GLUT4 tended to increase in preterm animals after a single dose of GCs but did not reach statistical significance, whereas GLUT4 increased in term counterparts. A limitation to our study is that a larger sample size may have led to statistical significance on differences in glucose transporters as they had a tendency to increase after exposure to GCs in preterm animals; unfortunately, due to the extremely high expenses for non-human primates, the sample size could not be increased.
GLUT4 is the predominant insulin-sensitive transporter in muscle. Since GLUT4 responds to insulin by promoting the intracellular transport of glucose, the increases we found in GLUT4 in term animals after GC exposure will likely play an important role in alterations in glucose homeostasis later in life. Consistent with our results, GLUT4 was found to be increased in skeletal muscle of fetal sheep exposed to dexamethasone in late gestation (Jellyman et al. 2012). Fetal hyperglycemia and hyperinsulinemia, along with perturbations in skeletal muscle glucose transporters of fetal sheep delivered in late gestation, have been found following a single course of GCs (Gray et al. 2006). While GLUT4 is the main glucose transporter involved in postprandial glucose transport, when circulating insulin concentrations are the highest, GLUT1 is responsible for the constitutive, insulin-independent glucose transport that takes place in all cells, including muscle (Pessin & Bell 1992). GLUT1 over-expression results in a three-to-fourfold increase in basal glucose transport in muscle ex vivo and improves glucose tolerance (Marshall et al. 1993), suggesting that GLUT1 also plays an important role in maintaining whole-body glucose homeostasis. Therefore, the significant changes seen only in preterm baboons with increased GLUT1 expression after GC exposure will likely improve glucose homeostasis in premature infants. Our findings indicate that alterations in glucose transporters differ in skeletal muscle of preterm and term baboons, which may explain the gestational differences in abnormal glucose homeostasis during early postnatal life.
A pitfall of this study is the larger proportion of males, as there are numerous reports of gender differences in various neonatal disease processes, which may have influenced the results (Long et al. 2013a). It is unlikely that the differences found in insulin-signaling between groups are due to gender, as all of the groups had a greater proportion of males with no gender differences between groups.
Recently, human studies showed that young adults born preterm, and exposed to antenatal GCs, had decreased aortic distensibility as well as reduction in beta cell function expressed by lower insulin, altered homeostasis model assessment, and higher glucose levels (Kelly et al. 2012). Therefore, it is extremely important to investigate the effects of GCs in glucose transporters and insulin-signaling pathways during critical periods of development as this therapy may have long-lasting consequences in multiple tissues.
In this study, we demonstrated that exposure to a single course of GCs during fetal life alters the insulin-signaling pathway in the muscle of fetal baboons in a manner dependent of gestation. The changes in some molecules take place in the same direction as those that occur at term with normal maturation. In several species, endogenous fetal corticosteroids increase as parturition approaches (Fowden 1998). Thus, the exposure to GCs may bring those normal changes forward, stimulating differentiation instead of proliferation and resulting in depressed growth and alterations of signaling pathways. Future studies will be needed to evaluate whether baboons exposed to GCs during fetal life have defects in the insulin-transduction pathways under insulin-stimulated conditions in the immediate postnatal period and later life.
Acknowledgments
FUNDING
This work was supported by grants from the Robert Wood Johnson Foundation (C.B.), UTHSCSA CTSA (UL1RR025767)(C.B.), American Diabetes Association (C.B. and N.M.), the National Institutes of Health (HL52636 to BPD resource Center, AG030979 to N.M.), P51RR13986 for facility support at the Southwest Foundation for Biomedical research, the San Antonio Nathan Shock Center (N.M), the UTHSCSA Executive Research Committee (N.M.), and the South Texas Health Research Center (N.M.).
We thank the personnel from the Veterinarian Services at UTHSCSA, the Oklahoma Primate Center, and the Texas Biomedical Research Institute for their dedication and support for this project.
Footnotes
Study was conducted at The University of Texas Health Science Center (UTHSC) in San Antonio, Texas, USA
DECLARATION OF INTEREST
There is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
AUTHOR CONTRIBUTIONS
C.B, P.N., and N.M. designed the study. D.A. and L.M. performed key experiments. C.B., A.M., L.M., D.A., P.N., and N.M. have participated in planning of the work, the interpretation of the results, and the writing of the paper. All authors have approved the manuscript.
References
- Ain R, Canham LN, Soares MJ. Dexamethasone-induced intrauterine growth restriction impacts the placental prolactin family, insulin-like growth factor-II and the Akt signaling pathway. The Journal of Endocrinology. 2005;185:253–263. doi: 10.1677/joe.1.06039. [DOI] [PubMed] [Google Scholar]
- Bae S, Cho H, Mu J, Birnbaum MJ. Isoform-specific regulation of insulin-dependent glucose uptake by Akt/protein kinase B. The Journal of Biological Chemistry. 2003;278:49530–49536. doi: 10.1074/jbc.M306782200. [DOI] [PubMed] [Google Scholar]
- Beardsall K, Vanhaesebrouck S, Ogilvy-Stuart AL, Vanhole C, Palmer CR, Ong K, vanWeissenbruch M, Midgley P, Thompson M, Thio M, et al. Prevalence and determinants of hyperglycemia in very low birth weight infants: cohort analyses of the NIRTURE study. The Journal of Pediatrics. 2010;157:715–719. e1–3. doi: 10.1016/j.jpeds.2010.04.032. [DOI] [PubMed] [Google Scholar]
- Blanco CL, Baillargeon JG, Morrison RL, Gong AK. Hyperglycemia in extremely low birth weight infants in a predominantly Hispanic population and related morbidities. Journal of perinatology: Official Journal of the California Perinatal Association. 2006;26:737–741. doi: 10.1038/sj.jp.7211594. [DOI] [PubMed] [Google Scholar]
- Blanco CL, Liang H, Joya-Galeana J, DeFronzo RA, McCurnin D, Musi N. The ontogeny of insulin signaling in the preterm baboon model. Endocrinology. 2010;151:1990–1997. doi: 10.1210/en.2009-0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco CL, McGill-Vargas LL, McCurnin D, Quinn AR. Hyperglycemia increases the risk of death in extremely preterm baboons. Pediatric Research. 2013;73:337–343. doi: 10.1038/pr.2012.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun T, Li S, Sloboda DM, Li W, Audette MC, Moss TJ, Matthews SG, Polglase G, Nitsos I, Newnham JP, Challis JR. Effects of maternal dexamethasone treatment in early pregnancy on pituitary-adrenal axis in fetal sheep. Endocrinology. 2009;150:5466–5477. doi: 10.1210/en.2009-0086. [DOI] [PubMed] [Google Scholar]
- Brownfoot FC, Crowther CA, Middleton P. Different corticosteroids and regimens for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database of Systematic Reviews (Online) 2008;(4):CD006764. doi: 10.1002/14651858.CD006764.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozinick JT, Roberts BR, Dohm GL. Defective signaling through Akt-2 and -3 but not Akt-1 in insulin-resistant human skeletal muscle: potential role in insulin resistance. Diabetes. 2003;52:935–941. doi: 10.2337/diabetes.52.4.935. [DOI] [PubMed] [Google Scholar]
- Calera MR, Martinez C, Liu H, Jack AK, Birnbaum MJ, Pilch PF. Insulin increases the association of Akt-2 with Glut4-containing vesicles. The Journal of Biological Chemistry. 1998;273:7201–7204. doi: 10.1074/jbc.273.13.7201. [DOI] [PubMed] [Google Scholar]
- Chavez AO, Lopez-Alvarenga JC, Tejero ME, Triplitt C, Bastarrachea RA, Sriwijitkamol A, Tantiwong P, Voruganti VS, Musi N, Comuzzie AG, DeFronzo RA, Folli F. Physiological and Molecular Determinants of Insulin Action in the Baboon. Diabetes. 2008;57:899–908. doi: 10.2337/db07-0790. [DOI] [PubMed] [Google Scholar]
- Cho H, Thorvaldsen JL, Chu Q, Feng F, Birnbaum MJ. Akt1/PKBalpha is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. The Journal of Biological Chemistry. 2001;276:38349–38352. doi: 10.1074/jbc.C100462200. [DOI] [PubMed] [Google Scholar]
- Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes & Development. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- Drake AJ, Liu L, Kerrigan D, Meehan RR, Seckl JR. Multigenerational programming in the glucocorticoid programmed rat is associated with generation-specific and parent of origin effects. Epigenetics: Official Journal of the DNA Methylation Society. 2011;6:1334–1343. doi: 10.4161/epi.6.11.17942. [DOI] [PubMed] [Google Scholar]
- Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consensus Development Panel on the Effect of Corticosteroids for Fetal Maturation on Perinatal Outcomes. JAMA: The Journal of the American Medical Association. 1995;273:413–418. doi: 10.1001/jama.1995.03520290065031. [DOI] [PubMed] [Google Scholar]
- Escobedo MB, Hilliard JL, Smith F, Meredith K, Walsh W, Johnson D, Coalson JJ, Kuehl TJ, Null DM, Robotham JL. A baboon model of bronchopulmonary dysplasia. I. Clinical features. Experimental and Molecular Pathology. 1982;37:323–334. doi: 10.1016/0014-4800(82)90045-4. [DOI] [PubMed] [Google Scholar]
- Fowden AL. The role of insulin in prenatal growth. Journal of Developmental Physiology. 1989;12:173–182. [PubMed] [Google Scholar]
- Fowden AL, Hughes P, Comline RS. The effects of insulin on the growth rate of the sheep fetus during late gestation. Quarterly Journal of Experimental Physiology (Cambridge, England) 1989;74:703–714. doi: 10.1113/expphysiol.1989.sp003322. [DOI] [PubMed] [Google Scholar]
- Fowden AL, Li J, Forhead AJ. Glucocorticoids and the preparation for life after birth: are there long-term consequences of the life insurance? The Proceedings of the Nutrition Society. 1998;57:113–122. doi: 10.1079/pns19980017. [DOI] [PubMed] [Google Scholar]
- Gray S, Stonestreet BS, Thamotharan S, Sadowska GB, Daood M, Watchko J, Devaskar SU. Skeletal muscle glucose transporter protein responses to antenatal glucocorticoids in the ovine fetus. The Journal of Endocrinology. 2006;189:219–229. doi: 10.1677/joe.1.06589. [DOI] [PubMed] [Google Scholar]
- Jellyman JK, Gardner DS, Edwards CM, Fowden AL, Giussani DA. Fetal cardiovascular, metabolic and endocrine responses to acute hypoxaemia during and following maternal treatment with dexamethasone in sheep. The Journal of Physiology. 2005;567:673–688. doi: 10.1113/jphysiol.2005.089805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellyman JK, Martin-Gronert MS, Cripps RL, Giussani DA, Ozanne SE, Shen QW, Du M, Fowden AL, Forhead AJ. Effects of cortisol and dexamethasone on insulin signalling pathways in skeletal muscle of the ovine fetus during late gestation. PloS One. 2012;7:e52363. doi: 10.1371/journal.pone.0052363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly BA, Lewandowski AJ, Worton SA, Davis EF, Lazdam M, Francis J, Neubauer S, Lucas A, Singhal A, Leeson P. Antenatal glucocorticoid exposure and long-term alterations in aortic function and glucose metabolism. Pediatrics. 2012;129:e1282–1290. doi: 10.1542/peds.2011-3175. [DOI] [PubMed] [Google Scholar]
- Liechty EA. The resistant premie: documenting the prevalence of hyperglycemia in the extremely low birth weight infant. The Journal of Pediatrics. 2010;157:699–700. doi: 10.1016/j.jpeds.2010.05.028. [DOI] [PubMed] [Google Scholar]
- Long NM, Ford SP, Nathanielsz PW. Multigenerational effects of fetal dexamethasone exposure on the hypothalamic-pituitary-adrenal axis of first- and second-generation female offspring. American Journal of Obstetrics and Gynecology. 2013a;208:217, e1–8. doi: 10.1016/j.ajog.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long NM, Smith DT, Ford SP, Nathanielsz PW. Elevated Glucocorticoids during Ovine Pregnancy increase Appetite and produce Glucose Dysregulation and Adiposity in Their Granddaughters in response to ad libitum feeding at one year of age. American Journal of Obstetrics and Gynecology. 2013b;209:353, e1–9. doi: 10.1016/j.ajog.2013.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louvi A, Accili D, Efstratiadis A. Growth-promoting interaction of IGF-II with the insulin receptor during mouse embryonic development. Developmental Biology. 1997;189:33–48. doi: 10.1006/dbio.1997.8666. [DOI] [PubMed] [Google Scholar]
- Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science (New York, NY) 2005;307:384–387. doi: 10.1126/science.1104343. [DOI] [PubMed] [Google Scholar]
- Marshall BA, Ren JM, Johnson DW, Gibbs EM, Lillquist JS, Soeller WC, Holloszy JO, Mueckler M. Germline manipulation of glucose homeostasis via alteration of glucose transporter levels in skeletal muscle. The Journal of Biological Chemistry. 1993;268:18442–18445. [PubMed] [Google Scholar]
- McCurnin D, Clyman RI. Effects of a patent ductus arteriosus on postprandial mesenteric perfusion in premature baboons. Pediatrics. 2008;122:e1262–1267. doi: 10.1542/peds.2008-2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitanchez-Mokhtari D, Lahlou N, Kieffer F, Magny JF, Roger M, Voyer M. Both relative insulin resistance and defective islet beta-cell processing of proinsulin are responsible for transient hyperglycemia in extremely preterm infants. Pediatrics. 2004;113:537–541. doi: 10.1542/peds.113.3.537. [DOI] [PubMed] [Google Scholar]
- Mizuno M, Siddique K, Baum M, Smith SA. Prenatal programming of hypertension induces sympathetic overactivity in response to physical stress. Hypertension. 2013;61:180–186. doi: 10.1161/HYPERTENSIONAHA.112.199356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newnham JP. Is prenatal glucocorticoid administration another origin of adult disease? Clinical and Experimental Pharmacology & Physiology. 2001;28:957–961. doi: 10.1046/j.1440-1681.2001.03559.x. [DOI] [PubMed] [Google Scholar]
- Ng PC. The effectiveness and side effects of dexamethasone in preterm infants with bronchopulmonary dysplasia. Archives of Disease in Childhood. 1993;68:330–336. doi: 10.1136/adc.68.3_spec_no.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozanne SE, Jensen CB, Tingey KJ, Storgaard H, Madsbad S, Vaag AA. Low birthweight is associated with specific changes in muscle insulin-signalling protein expression. Diabetologia. 2005;48:547–552. doi: 10.1007/s00125-005-1669-7. [DOI] [PubMed] [Google Scholar]
- Pessin JE, Bell GI. Mammalian facilitative glucose transporter family: structure and molecular regulation. Annual Review of Physiology. 1992;54:911–930. doi: 10.1146/annurev.ph.54.030192.004403. [DOI] [PubMed] [Google Scholar]
- Philipps AF, Rosenkrantz TS, Clark RM, Knox I, Chaffin DG, Raye JR. Effects of fetal insulin deficiency on growth in fetal lambs. Diabetes. 1991;40:20–27. doi: 10.2337/diab.40.1.20. [DOI] [PubMed] [Google Scholar]
- Schwartz R, Teramo KA. Effects of diabetic pregnancy on the fetus and newborn. Seminars in Perinatology. 2000;24:120–135. doi: 10.1053/sp.2000.6363. [DOI] [PubMed] [Google Scholar]
- Selak MA, Storey BT, Peterside I, Simmons RA. Impaired oxidative phosphorylation in skeletal muscle of intrauterine growth-retarded rats. American Journal of Physiology. Endocrinology and Metabolism. 2003;285:e130–137. doi: 10.1152/ajpendo.00322.2002. [DOI] [PubMed] [Google Scholar]
- Walker KS, Deak M, Paterson A, Hudson K, Cohen P, Alessi DR. Activation of protein kinase B beta and gamma isoforms by insulin in vivo and by 3-phosphoinositide-dependent protein kinase-1 in vitro: comparison with protein kinase B alpha. The Biochemical Journal. 1998;331:299–308. doi: 10.1042/bj3310299. [DOI] [PMC free article] [PubMed] [Google Scholar]