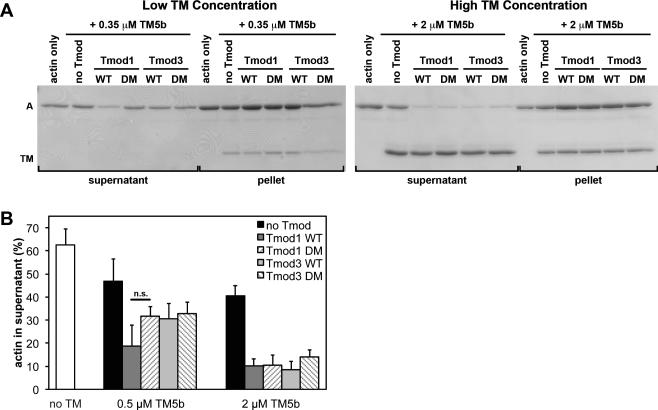
Figure 5. Effects of wild-type and TM-binding-disabled mutant Tmod proteins on LatA-induced depolymerization from the pointed ends of CapZ-capped actin filaments coated with TM5b.
(A) CapZ-capped actin filaments (CapZ:actin ratio of 1:100) were incubated with TM5b then mixed with wild-type Tmod1, wild-type Tmod3, Tmod1-L27G/I131D (DM), or Tmod3-L29G/L134D (DM), diluted five-fold and incubated in the presence of 10 μM LatA to depolymerize the F-actin. Final concentrations of Tmods and TMs were as indicated, with 20 nM CapZ-capped actin filaments (2 μM actin). The mixtures were ultracentrifuged, fractionated into supernatants and pellets, and separated using SDS-PAGE. A, actin; TM; tropomyosin. (B) Densitometric quantification was performed to calculate the percentage of depolymerized actin in the supernatants. Data shown are mean ± S.D. of three independent experiments. n.s., not significant (Student's t-test).