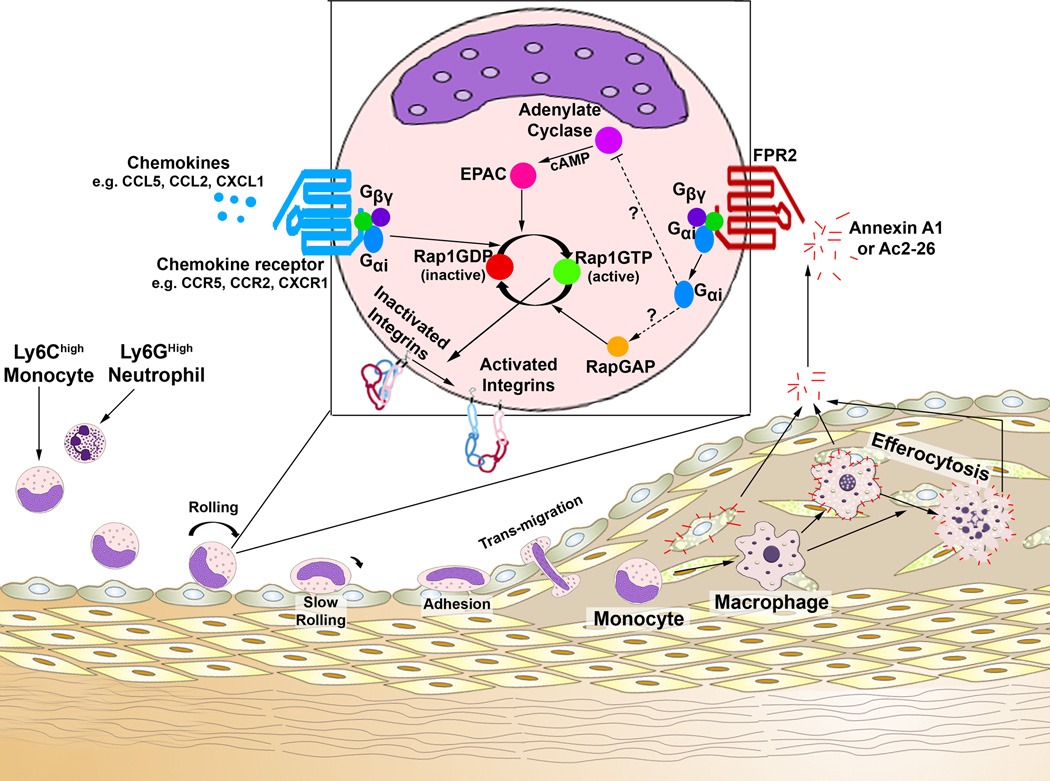
Figure 1. Potential mechanisms of action for how Annexin A1/FPR2 might antagonize integrin activation and avidity.
The recruitment of myeloid cells to atherosclerotic plaques represents a critical process in the initiation and progression of atherosclerosis. In this issue of Circulation Research, Dreshler and colleagues9 investigated the actions of Annexin A1, the Annexin A1 N-terminal peptide AC2-26, and its receptor, N-Formyl peptide receptor 2 (FPR2), on atherosclerosis in Apoe−/− mice. Ly6Chigh monocytes and Ly6Ghigh neutrophils (left) use several chemokine receptors and leukocyte adhesion molecules, in the steps of the adhesion cascade (rolling to transmigration), to migrate to atherosclerotic plaques. Importantly, in order for monocytes or neutrophils to roll, adhere, and transmigrate, membrane integrins need to activate and properly cluster, through outside-in activation, inside-out activation, or changes in avidity. This study demonstrates that Annexin A1, which is primarily present within lesional Mac2+ macrophages and foam cells, may be released from the plaque and act on circulating FPR2+ monocytes and neutrophils to antagonize integrin activation, clustering, and therefore migration. How does Annexin A1/FPR2 signaling work? While the full pathway was not examined, the pathway at least involves activation of the small GTPase Rap1 and integrin activation. So how might Annexin A1/FPR2 signaling impact Rap1-activation? FPR2 is a known G-protein coupled receptor and has been reported to associate with Gαi2, Gβ, and Gγ. Thus while the exact signaling mechanisms involved are unclear, several pathways could be involved. Annexin A1-FPR2-Gαi2 signaling might serve to antagonize Adenylate Cyclase, thereby lowering cyclic AMP levels, and antagonizing EPAC proteins, which are known guanine exchange factor for Rap1. These actions would result in a decrease in GTP-Rap1 dependent integrin activation. Alternatively, Annexin A1-FPR2-Gαi2 activation might promote the recruitment and activation of a RapGAP, which would promote hydrolysis of GTP and thereby deactivate Rap1; achieving the same end. All together, while additional work on the mechanisms of action is necessary, the results presented by Drescher and colleagues9 indicate that Annexin A1-might serve as an endogenous negative regulator of myeloid cell recruitment in early atherogenesis.