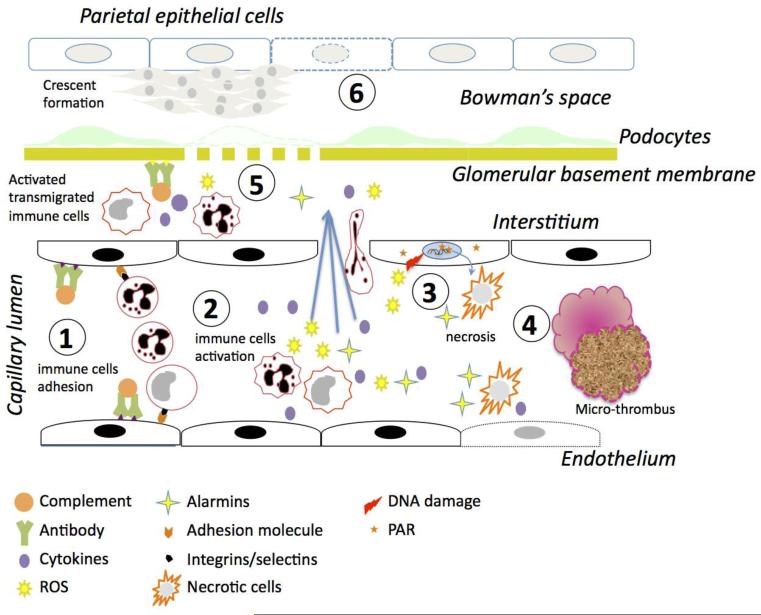
Figure 3. Role of necrosis in renal tissue injury.
1. Deposition of immune complexes on the endothelial cells fixes complement and allows for the adherence and activation of monocytes and neutrophils through interactions between adhesion molecules and selectins/integrins. 2. Activation of monocytes and neutrophils leads to release of pro-inflammatory cytokines, reactive oxygen species (ROS) activate endothelial cells and lead to endothelial dysfunction. The neutrophils and monocytes then migrate into the interstitium. 3. The ROS generated causes DNA damage, leading to PARP-1 activation and necrosis of both leukocytes and endothelial cells. The necrotic cells release alarmins further activating leukocytes and endothelial cells. 4. Endothelial dysfunction, leukocyte infiltration, and necrosis finally lead to micro-thrombi causing areas of local ischemia. Necrosis resulting from all these processes leads to a feed back loop that exacerbates the inflammatory response and induces further tissue damage. 5. The leukocytes migrated into the interstitium get further activated by the immune complexes deposited on the glomerular basement membranes. Cytokines and inflammatory mediators released by the immune cells in the interstitium cause glomerular membrane rupture and loss of podocyte foot processes. 6. The inflammatory mediators enter the Bowman's space, activate epithelial cells and finally lead to crescent formation.